Novel crystal form of cefamandole nafate compound and crystal preparation method thereof
A technology for cefamandole sodium and compound, which is applied in the field of pharmaceutical separation, can solve the problems of unsolved solubility, unstable crystal water, many insoluble particles, etc., achieves easy commercial industrialization scale implementation, is conducive to long-term storage, and has high fluidity. Good results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
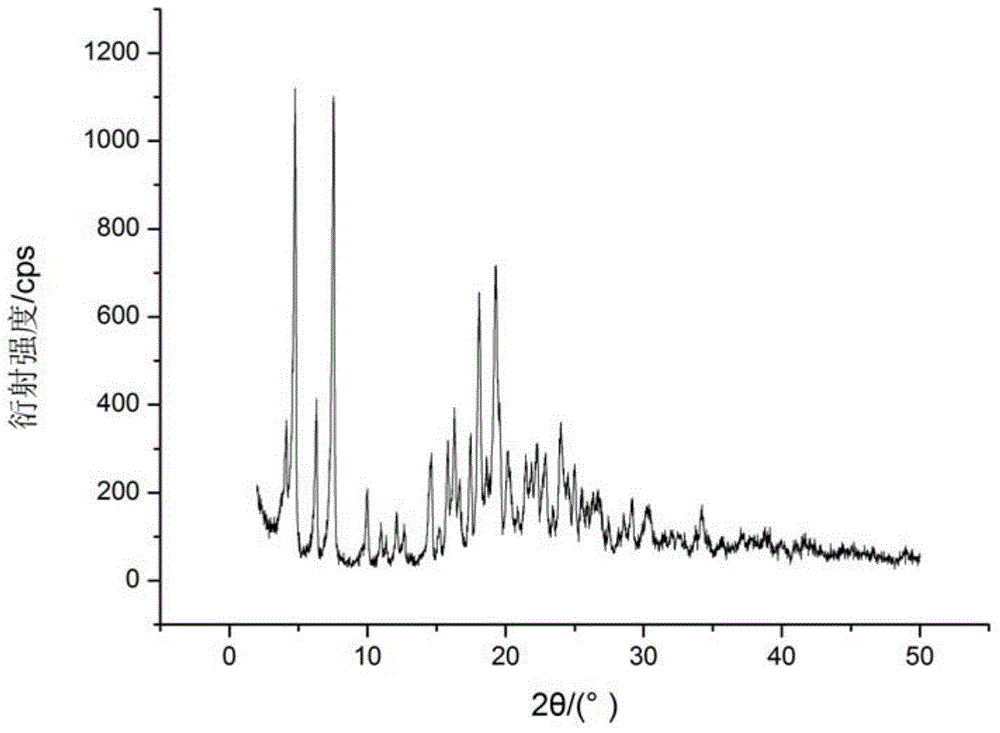
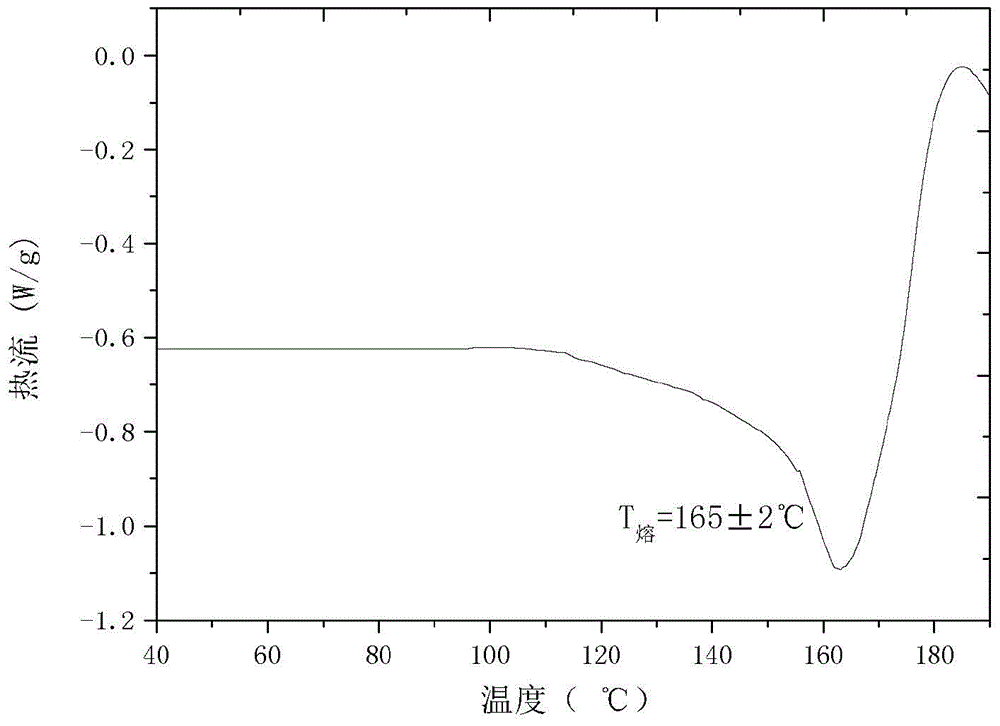
[0023] 0.40g of dry cefmendol sodium solid was added to 10mL of 1,4-dioxane to form a suspension, the suspension was stirred at a stirring rate of 600r / min, and the suspension was heated to 40°C, constant Temperature, stir the suspension for 5h, then cool the suspension to 5℃ at a rate of 0.2℃ / min, stir the suspension for 5h at a constant temperature, vacuum filter the crystal slurry, dry the product at 20℃, normal pressure for 6h to constant The new crystal form of cefmendol sodium was obtained. The X-ray powder diffraction pattern of the product is at diffraction angle 2θ=4.01, 4.66, 6.18, 7.47, 9.95, 10.70, 14.56, 15.82, 16.26, 17.40, 18.05, 19.26, 20.15, 21.45, 22.25, 22.78, 24.00, 24.94, 30.17 , There is a characteristic peak at 34.16°C, and DSC has an endothermic peak at 164°C. The new crystal form product obtained by this method has a melting point about 69°C higher than that of the common crystal form, and has higher thermal stability. It has no degradation and changes...
Embodiment 2
[0025] Add 0.43 g of dry cefmendol sodium solid to 4 mL of methanol to form a suspension, stir the suspension at a stirring rate of 800 r / min, and heat the suspension to 45° C., at a constant temperature, and stir the suspension for 8 hours. Then the temperature of the suspension was lowered to 10℃ at a rate of 1℃ / min, the suspension was stirred for 9h at a constant temperature, the crystalline slurry was vacuum filtered, and the product was dried at 40℃ and normal pressure for 10h to constant weight to obtain cefmandol Sodium crystal products. The X-ray powder diffraction pattern of the product is at diffraction angle 2θ=4.04, 4.70, 6.22, 7.48, 9.90, 10.80, 14.66, 15.72, 16.22, 17.38, 18.02, 19.20, 20.08, 21.38, 22.12, 22.82, 23.88, 24.92, 30.32 , There is a characteristic peak at 34.16°C, and the DSC analysis chart has an endothermic peak at 166°C. The new crystal form product obtained by this method has a melting point about 71°C higher than that of the common stable crysta...
Embodiment 3
[0027] Add 0.50 g of dry cefmendol sodium solid to 10 mL of ethyl acetate to form a suspension, stir the suspension at a stirring rate of 1000 r / min, and heat the suspension to 48° C., at a constant temperature, and stir the suspension After 9 hours, the temperature of the suspension was lowered to 15°C at a rate of 1°C / min, the suspension was stirred at a constant temperature for 8 hours, and the crystal slurry was vacuum filtered. The product was dried at 60°C and normal pressure for 10 hours to a constant weight to obtain cefmeng Polyester sodium crystal product. The X-ray powder diffraction pattern of the product is at diffraction angle 2θ = 4.10, 4.76, 6.28, 7.54, 9.98, 10.61, 14.46, 15.62, 16.30, 17.46, 18.08, 19.28, 20.16, 21.48, 22.26, 22.84, 24.00, 24.98, 30.26 , There is a characteristic peak at 34.22°C, and the DSC analysis chart has an endothermic peak at 164°C. The new crystal form product obtained by this method has a melting point of about 69°C higher than that ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com