Method for preparing cefamandole nafate sodium powder injection preparation for injection
A technology for mendolate sodium powder and injection, which is applied in the field of preparation of cefamandole sodium powder for injection, to achieve the effects of quality optimization, improvement of reaction efficiency, and change of crystallization mode
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
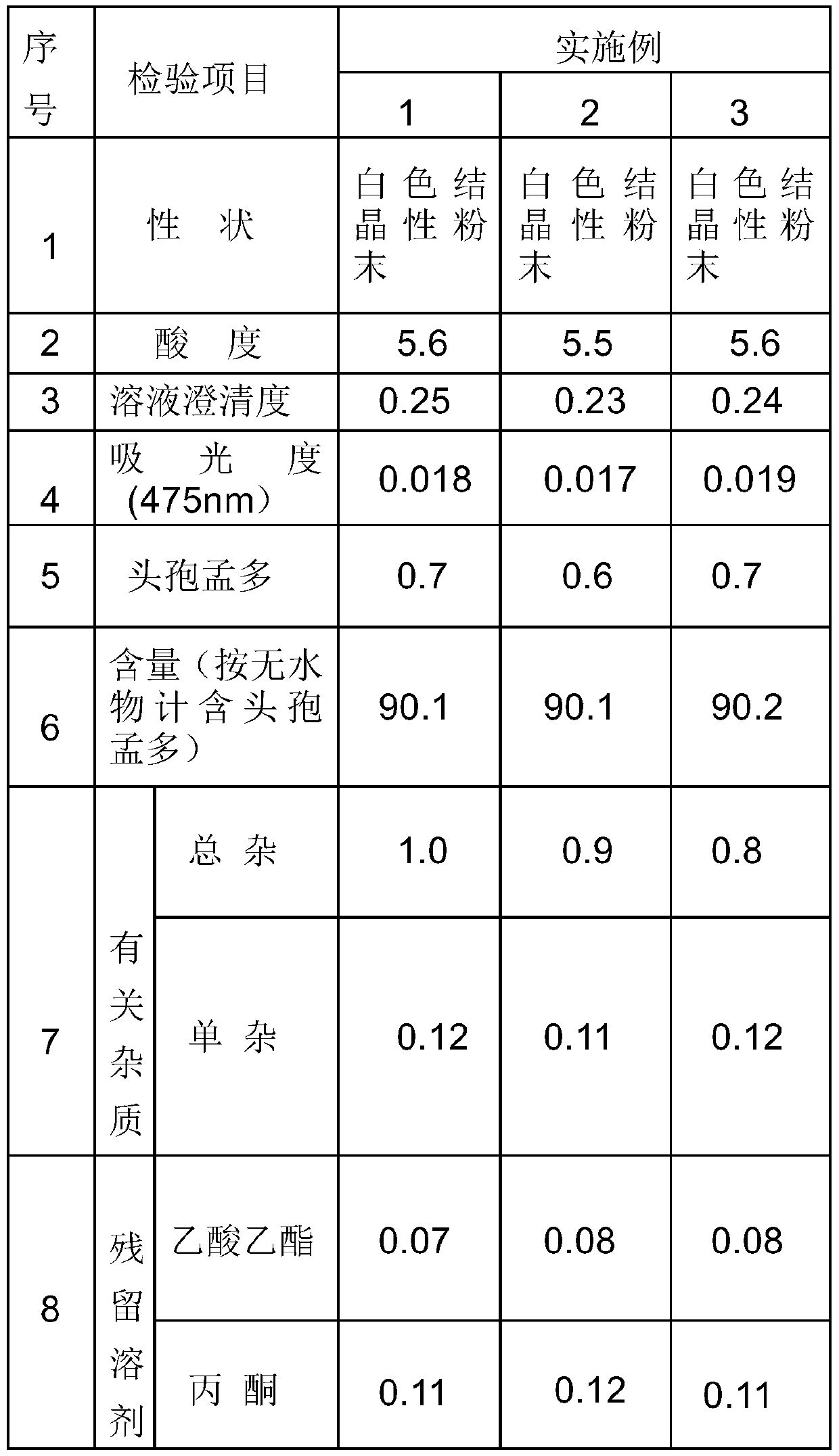
Embodiment 1
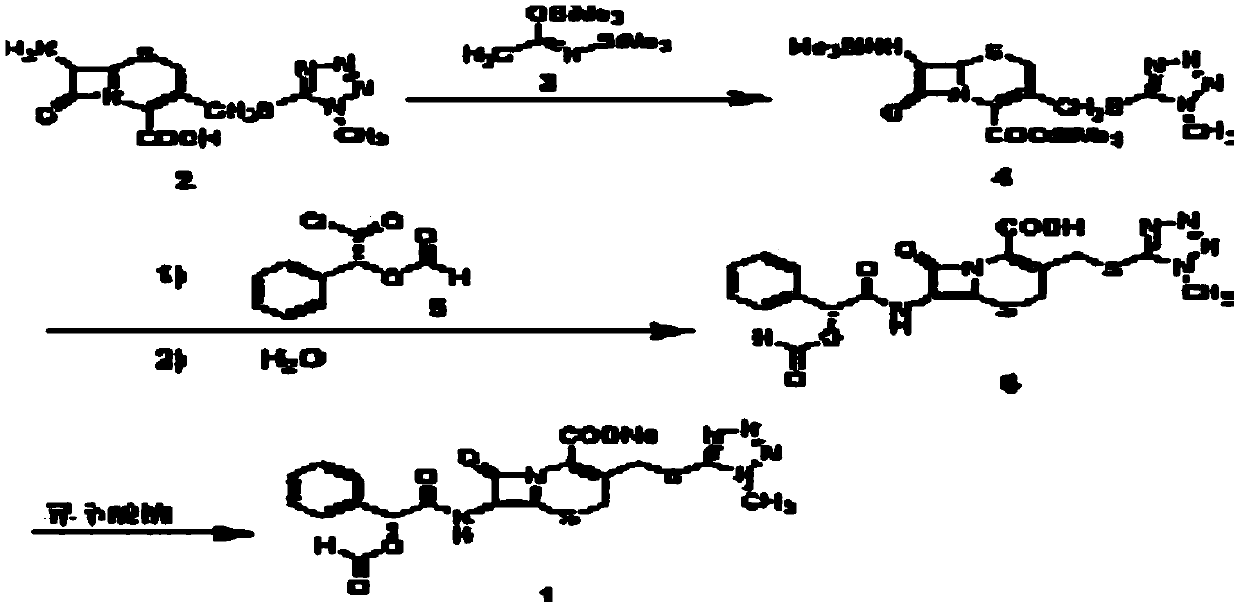
[0044] ①Dissolution of N,N-dimethylaniline: Add 14ml of N,N-dimethylaniline into 25ml of dichloromethane at -10°C, stir well and keep at -10°C for later use;
[0045] ②Dissolution of D-formylmandelic acid chloride: Add 18ml of D-formylmandelic acid chloride to a solution of 25ml of dichloromethane at -10°C, stir well and keep at -10°C for later use;
[0046] ③Silylation reaction: Add 30g of 7-TMCA and 340ml of dichloromethane to a 1000ml reaction bottle and stir evenly. When the system temperature is 12°C, add 20ml of hexamethyldisilamine to the above solution, and return Clarification, the reflux temperature is 40°C, reflux reaction for 1.5h, and the temperature of the system is lowered to -10°C to obtain the reaction solution 1;
[0047] ④ Synthesis of cefamandoleate: within 1.2 hours, slowly add dropwise the N, N-dimethylaniline dichloromethane solution in step ① and the D-formylmandelic acid in step ② in the reaction solution 1 Acyl chloride dichloromethane solution to ob...
Embodiment 2
[0053] ①Dissolution of N,N-dimethylaniline: Add 14ml of N,N-dimethylaniline into 25ml of dichloromethane at -10°C, stir well and keep at -12°C for later use;
[0054] ②Dissolution of D-formylmandelic acid chloride: Add 18ml of D-formylmandelic acid chloride to a solution of 25ml of dichloromethane at -10°C, stir well and keep at -13°C for later use;
[0055] ③Silylation reaction: Add 50g of 7-TMCA and 570ml of dichloromethane to a 1000ml reaction bottle and stir evenly. When the system temperature is 15°C, add 40ml of hexamethyldisilamine to the above solution, and return Clarification, the reflux temperature is 41°C, the reflux reaction is 1.7h, and the temperature of the system is lowered to -10°C to obtain the reaction solution 1;
[0056] ④ Synthesis of cefamandoleate: within 1.5 hours, slowly add dropwise the N, N-dimethylaniline dichloromethane solution in step ① and the D-formylmandelic acid in step ② in the reaction solution 1 Acyl chloride dichloromethane solution to...
Embodiment 3
[0062] ①Dissolution of N,N-dimethylaniline: Add 30ml of N,N-dimethylaniline into 54ml of dichloromethane at -15°C, stir well and keep at -15°C for later use;
[0063] ②Dissolution of D-formylmandelic acid chloride: Add 18ml of D-formylmandelic acid chloride to a solution of 49ml of dichloromethane at -10°C, stir well and keep at -15°C for later use;
[0064] ③Silylation reaction: Add 45g of 7-TMCA and 500ml of dichloromethane to a 1500ml reaction bottle and stir evenly. When the system temperature is 17°C, add 30ml of hexamethyldisilamine to the above solution and return to Clarification, the reflux temperature is 42°C, reflux reaction for 2h, and the temperature of the system is lowered to -10°C to obtain the reaction solution 1;
[0065] ④ Synthesis of cefamandoleate: within 1.7 hours, slowly add dropwise the N,N-dimethylaniline dichloromethane solution in step ① and the D-formylmandelic acid in step ② in the reaction solution 1 Acyl chloride dichloromethane solution to obt...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com