Novel crystalline form of cefamandole nafate compound, preparation and preparing method thereof
a cefamandole nafate and compound technology, applied in the field of medicine separation technology, can solve the problems of uncertain security risks of lidocaine and reduced glutathione active drugs, and achieve the effects of improving thermal stability, improving form, and high melting poin
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
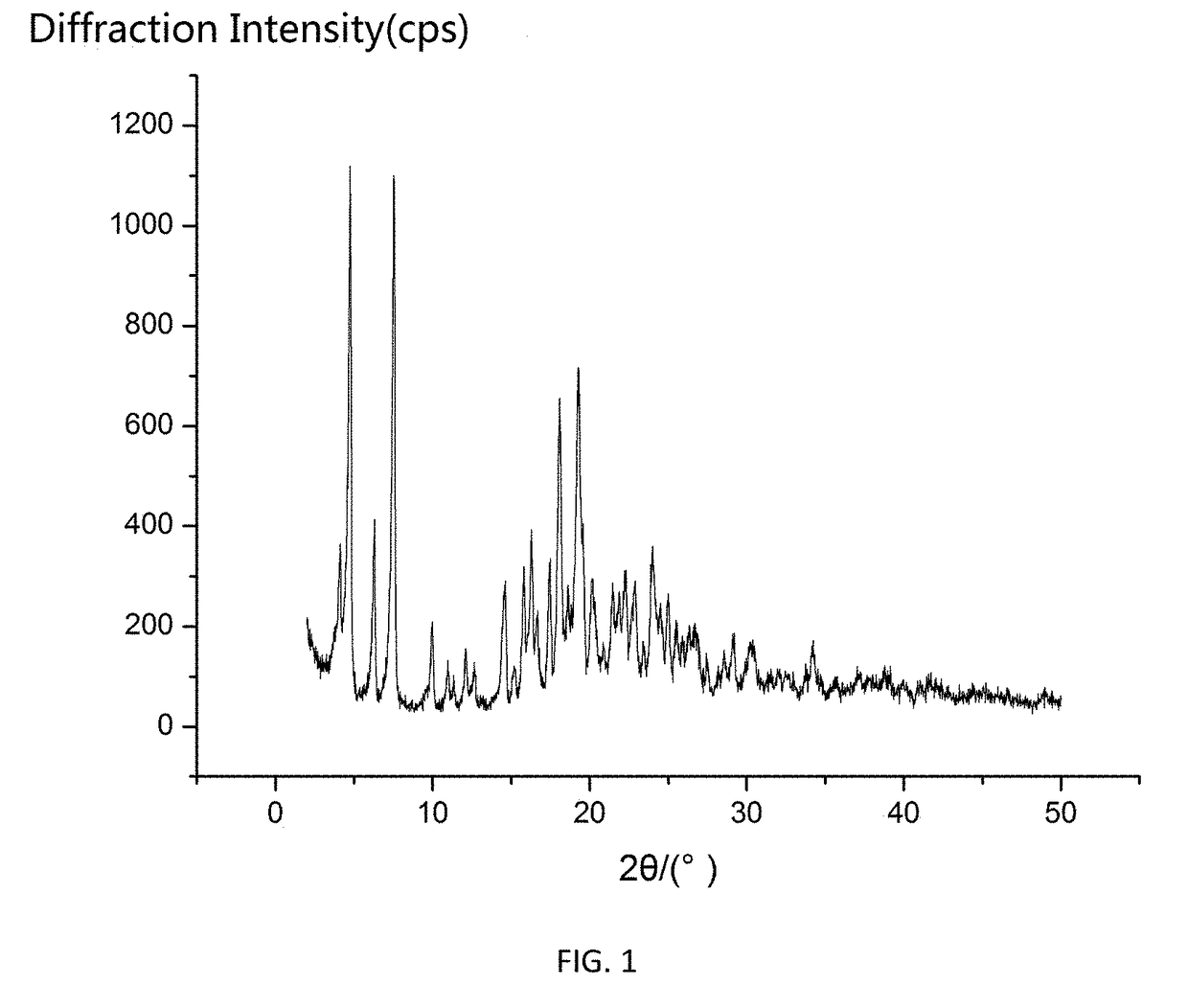
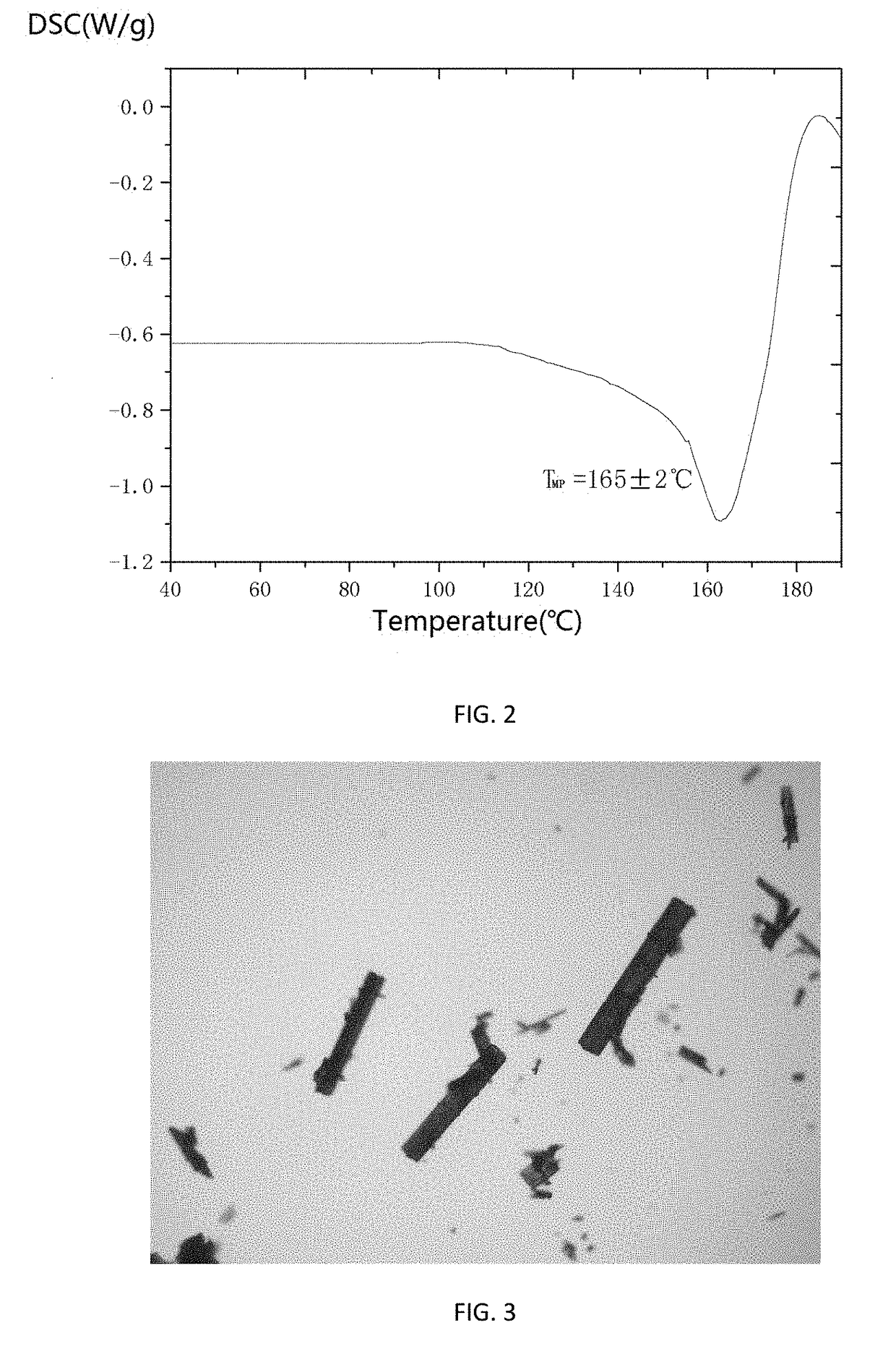
[0024]0.40 g of Cefamandole Nafate as dried solid was added to 10 mL of 1,4-dioxane to form a suspension, stirring the suspension at 600 r / min and heating to 40° C., continuing to stir for 5 h under constant temperature, and then cooling the suspension down to 5° C. at a cooling rate of 0.2° C. / min and stirring for 5 h under constant temperature, vacuum filtrating the crystal slurry, and the residue was dried at 20° C. and under normal pressure for 6 h to constant weight, to obtain a product of novel crystalline form of Cefamandole Nafate. The XRD pattern of the product was shown in FIG. 1, having characteristic peaks expressed in degrees 2θ at 4.01, 4.66, 6.18, 7.47, 9.95, 10.70, 14.56, 15.82, 16.26, 17.40, 18.05, 19.26, 20.15, 21.45, 22.25, 22.78, 24.00, 24.94, 30.17, and 34.16. The DSC thermogram was shown in FIG. 2, having an endothermic peak at 164° C. The product of the novel crystalline form provided by this method had a melting point, which is about 69° C. higher than that o...
example 2
[0025]0.43 g of Cefamandole Nafate as dried solid was added to 4 mL of methanol to form a suspension, stirring the suspension at 800 r / min and heating to 45° C., continuing to stir for 8 h under constant temperature, and then cooling the suspension down to 10° C. at a cooling rate of 1° C. / min and stirring for 9 h under constant temperature, vacuum filtrating the crystal slurry, and the residue was dried at 40° C. and under normal pressure for 10 h to constant weight, to obtain a product of novel crystalline form of Cefamandole Nafate. The XRD pattern of the product had characteristic peaks expressed in degrees 2θ at 4.04, 4.70, 6.22, 7.48, 9.90, 10.80, 14.66, 15.72, 16.22, 17.38, 18.02, 19.20, 20.08, 21.38, 22.12, 22.82, 23.88, 24.92, 30.32, 34.16. The DSC thermogram had an endothermic peak at 166° C. The product of the novel crystalline form provided by this method had a melting point, which is about 71° C. higher than that of a common crystal form, with higher thermal stability, ...
example 3
[0026]0.50 g of Cefamandole Nafate as dried solid was added to 10 mL of ethyl acetate to form a suspension, stirring the suspension at 1000 r / min and heating to 48° C., continuing to stir for 9 h under constant temperature, and then cooling the suspension down to 15° C. at a cooling rate of 1° C. / min and stirring for 8 h under constant temperature, vacuum filtrating the crystal slurry, and the residue was dried at 60° C. and under normal pressure for 10 h to constant weight, to obtain a product of novel crystalline form of Cefamandole Nafate. The XRD pattern of the product had characteristic peaks expressed in degrees 2θ at 4.10, 4.76, 6.28, 7.54, 9.98, 10.61, 14.46, 15.62, 16.30, 17.46, 18.08, 19.28, 20.16, 21.48, 22.26, 22.84, 24.00, 24.98, 30.26, 34.22. The DSC thermogram had an endothermic peak at 164° C. The product of the novel crystalline form provided by this method had a melting point, which is about 69° C. higher than that of a common crystal form, with higher thermal stab...
PUM
| Property | Measurement | Unit |
|---|---|---|
| time | aaaaa | aaaaa |
| stirring time | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com