Cefamandole nafate hydrate and preparation method thereof
A technology of cefamandole sodium and hydrate, applied in the field of medicine, can solve the problems of inconvenient use and large amount of production, and achieve the effects of good stability, fast dissolution speed and smooth surface
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment example 1
[0013] Take by weighing 200g of cefamandole sodium raw material, place in a 500ml beaker, add 50ml of water to dissolve. Slowly add (completely add within half an hour) 200ml of acetone, stir while adding, control the temperature to 2-8°C, and keep the temperature constant for 3 hours.
[0014] Suction filtration, wash the filter cake with ethanol, and dry under reduced pressure at 50°C for 4 hours to obtain cefamandole sodium pentahydrate.
Embodiment example 2
[0016] Take by weighing 200g of cefamandole sodium raw material, place in a 500ml beaker, add 50ml of water to dissolve. Slowly add (completely add within half an hour) 200ml of eluent, stirring while adding, ethanol 50ml, ethyl acetate 150ml, control the temperature to 2-8°C, and keep the temperature constant for 3 hours.
[0017] Suction filtration, wash the filter cake with ethanol, and dry under reduced pressure at 50°C for 4 hours to obtain cefamandole sodium pentahydrate.
Embodiment example 3
[0019] Take cefamandole sodium raw material 2000g, place in the 5000ml beaker, add 500ml water to dissolve. Add slowly (within 40 minutes) 2000ml of eluting agent, stir while adding, eluting agent is 500ml of dichloromethane, 1500ml of ethyl acetate, control the temperature to 2-8°C, and keep the temperature constant for 3 hours.
[0020] Suction filtration, wash the filter cake with ethanol, and dry under reduced pressure at 50°C for 4 hours to obtain cefamandole sodium pentahydrate.
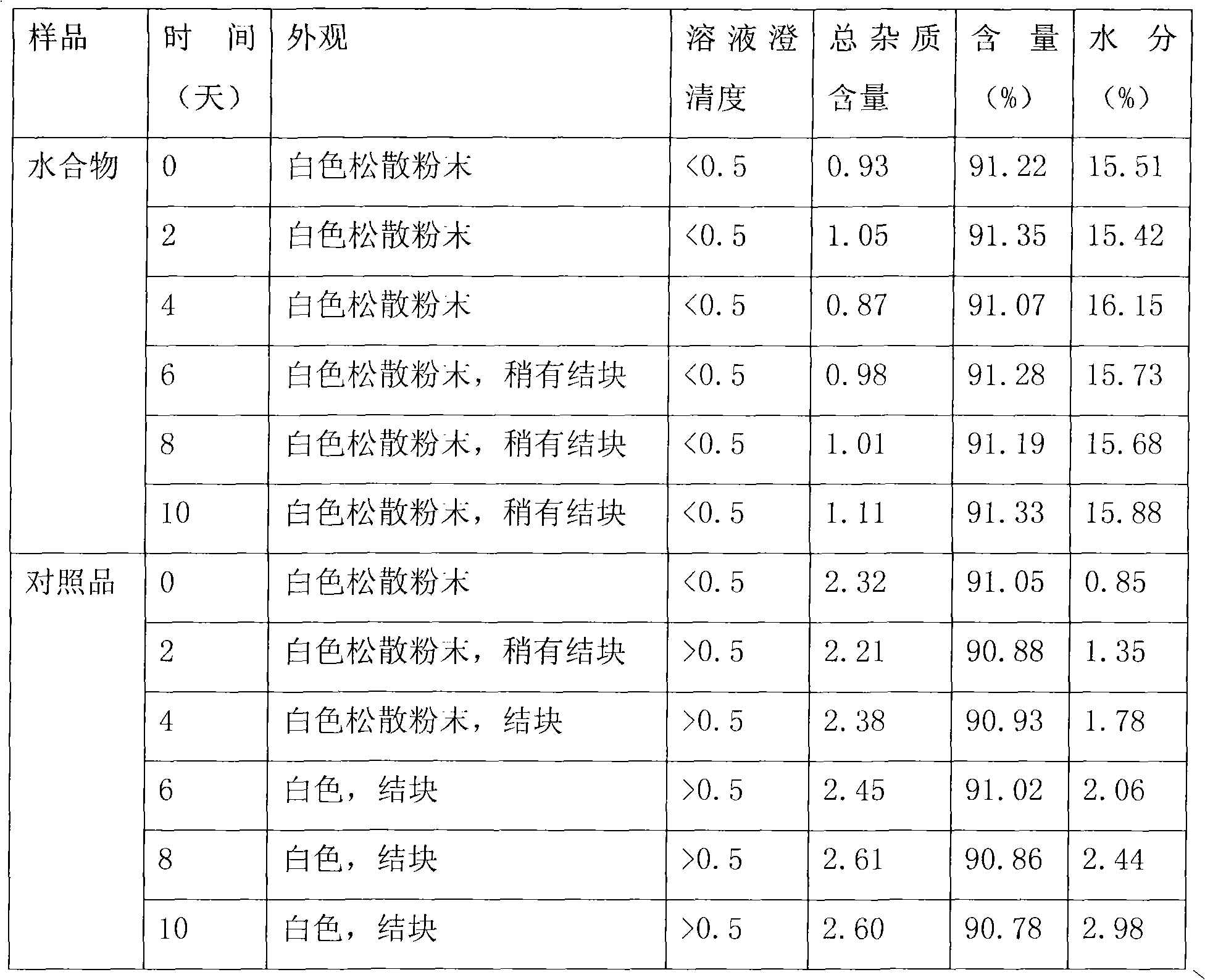
[0021] Research on the relevant characteristics of cefamandole sodium hydrate:
[0022] 1. Elemental analysis
[0023] Get the cefamandole sodium hydrate prepared above and carry out elemental analysis, the result: C 37.88%; H 4.50%; N 13.87%, agree with the cefamandole sodium pentahydrate theoretical value (theoretical value: C 37.84%; H 4.48%; N 13.94%)
[0024] 2. Differential thermal analysis
[0025] The above-prepared cefamandole sodium hydrate was subjected to differential thermal an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com