Cefamandole nafate new crystal form and crystallization preparing method thereof
A technology of cefamandole sodium and crystal form, which is applied in the field of new crystal form of cefamandole sodium and its preparation, can solve problems such as unsuitable for popularization and use, hidden safety hazards, poor stability, etc., and achieve easy commercial industrialization scale Implementation, good for long-term storage, good thermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
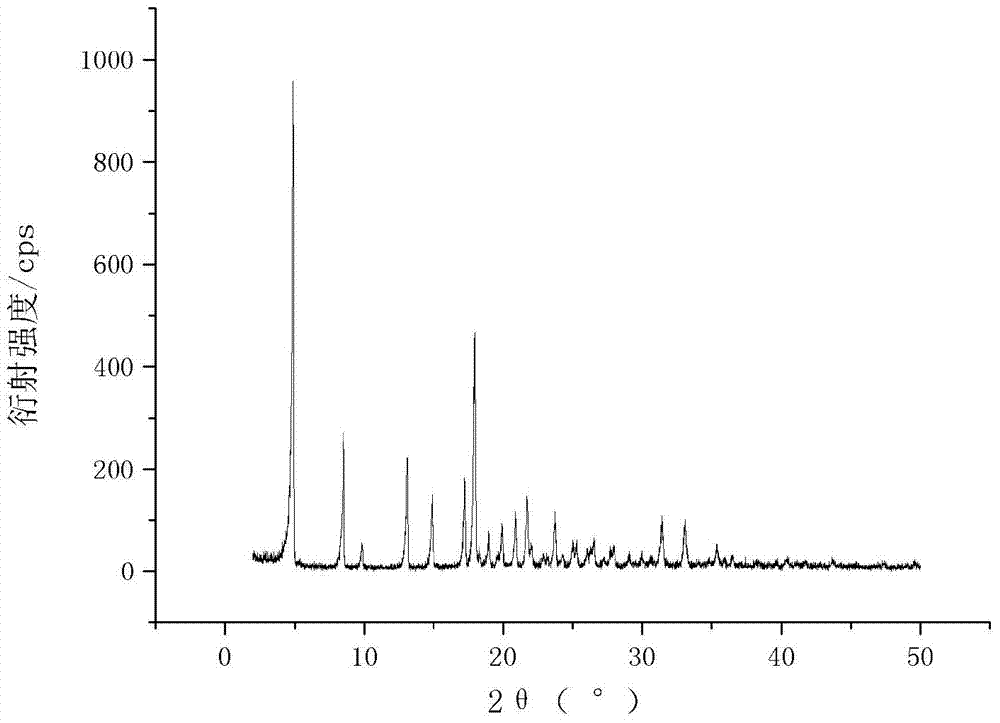
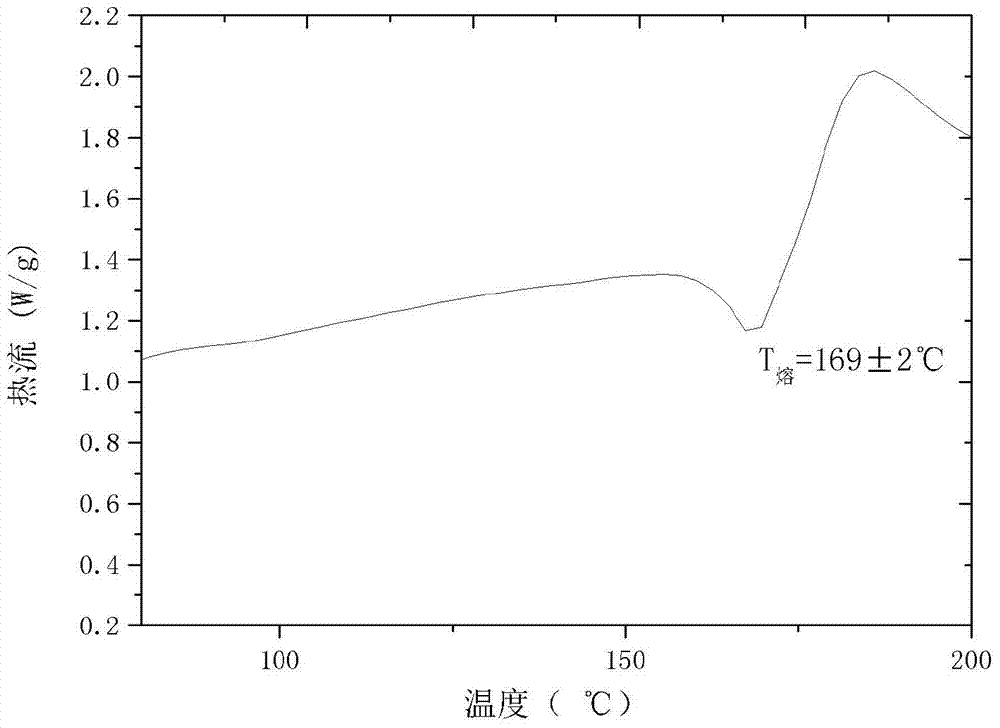
[0023] Add 2.50 g of dry cefamandole sodium solid into 5 mL of water and propionic acid mixed solution to form a suspension, wherein the volume ratio of water to propionic acid is 3:2, stir the suspension at 10°C to dissolve all the solids , constant temperature, dropwise add 25mL of acetone to the solution at a rate of 0.05ml / min, then cool down to 0°C at a rate of 0.05°C / min, let the crystal grow for 4h, filter and place the obtained wet product at 10°C at room temperature Dry under high pressure for 3 hours to obtain a new crystal product of cefamandole sodium. The X-ray powder diffraction pattern of the new crystal product is as follows: figure 1 As shown, it has characteristic peaks at diffraction angles 2θ=4.8, 8.5, 13.1, 14.5, 17.2, 18.2, 19.1, 19.6, 21.0, 21.6, 22.0, 23.4, 25.2, 31.1 degrees; the DSC of the new crystal form product is as follows figure 2 As shown, it has a characteristic endothermic peak at 169.25 °C. The melting point of the new crystalline product...
Embodiment 2
[0025] Add 2.00 g of dry cefamandole sodium solid into 3 ml of propionic acid to form a suspension, stir the suspension at 30°C to dissolve all the solids, and add 100 mL of ethanol dropwise to the solution at a rate of 20 ml / min at a constant temperature , then lower the temperature to 2°C at a rate of 1°C / min, let the crystal grow for 15 hours, filter and dry the obtained wet product at 10°C under normal pressure for 5 hours to obtain a new crystal form of cefamandole sodium. The X-ray powder diffraction pattern of the new crystalline product has characteristic peaks at diffraction angles 2θ=4.9, 8.3, 13.0, 14.5, 17.3, 18.1, 19.0, 19.7, 21.1, 21.7, 22.2, 23.4, 25.2, 31.0 degrees, DSC There is a characteristic endothermic peak at 169.20°C. The melting point of the new crystalline product obtained by this method is higher than that of the crystalline forms reported in all patents. It has high thermal stability, no degradation changes after long-term storage, and the appearance...
Embodiment 3
[0027] Add 2.00 g of dry cefamandole sodium solid into 2.5 ml dimethyl sulfoxide to form a suspension, stir the suspension at 30 ° C to dissolve all the solids, and add the solid to the solution at a constant temperature at a rate of 20 ml / min. Add 100mL of isopropanol dropwise, then cool down to 5°C at a rate of 1°C / min, let the crystal grow for 5h, filter and dry the obtained wet product at 10°C under normal pressure for 3h to obtain cefamandole sodium new Crystalline products. The X-ray powder diffraction pattern of the new crystalline product has characteristic peaks at diffraction angles 2θ=4.7, 8.2, 13.1, 14.4, 17.2, 18.2, 19.1, 19.6, 21.2, 21.7, 22.1, 23.3, 25.2, 31.0 degrees, DSC There is a characteristic endothermic peak at 168.05°C. The melting point of the new crystalline product obtained by this method is higher than that of the crystalline forms reported in all patents. It has high thermal stability, no degradation change after long-term storage, the appearance o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com