Process for synthesizing cefodizime sodium through enzyme method
A technology for cefodizime sodium and enzymatic synthesis, applied in the directions of organic chemistry, fermentation, etc., can solve the problems of complicated ceftizime sodium process, etc., and achieve the effects of easy separation, high yield, and convenient transportation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
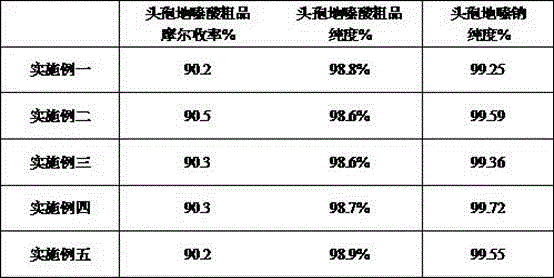
[0031] In the reactor, 3900 u of immobilized cefodizime synthase was added to 130 mL of deionized water, and the stirring was started. 0.04 mol of TACA and 0.046 mol of methyl thioxamic acid were added. Sodium hydroxide was added to adjust the reaction pH to 6.8. Keep the reaction temperature at 18~20°C. React for 90 minutes. The reaction solution was separated from the immobilized cefodizime synthase. The resulting product was adjusted to pH 3.2 with 15% hydrochloric acid, filtered, washed, and dried to obtain the crude product of cefodizime. The crude product of cefodizime was dissolved in a mixed solvent of 100 mL of ethanol and 100 mL of acetone. Dissolve 12g in 25mL ethanol and 25mL acetone mixed solvent, slowly drop it into the above mixed solvent, stir slowly until cloudy and crystals appear, add dropwise 100mL of toluene, grow crystals at 10°C for 1 hour, filter with suction, wash with 20mL of toluene, vacuum Dried to get cefodizime sodium.
Embodiment 2
[0033] In the reactor, 2600 u of immobilized cefodizime synthase was added to 130 mL of deionized water, and the stirring was started. 0.04 mol of TACA and 0.048 mol of methyl athioxamate were added. Sodium hydroxide was added to adjust the reaction pH to 7.0. The reaction temperature was maintained at 13-15°C. Reaction 140min. The reaction solution was separated from the immobilized cefodizime synthase. The obtained product was adjusted to pH 3.7 with 15% hydrochloric acid, filtered, washed, and dried to obtain crude cefodizime acid. Dissolve the crude product of cefodizime in a mixed solvent of 80mL ethanol and 80mL acetone, dissolve 15g of sodium isooctyl alcohol in a mixed solvent of 30mL ethanol and 30mL acetone, slowly drop it into the above mixed solvent, and slowly stir until cloudy After crystallization, add 80 mL of toluene dropwise, grow the crystal at 10°C for 1 hour, filter with suction, wash with 20 mL of toluene, and dry in vacuo to obtain cefodizime sodium....
Embodiment 3
[0035] In the reactor, 3000 u of immobilized cefodizime synthase was added to 130 mL of deionized water, and the stirring was started. 0.04 mol of TACA and 0.044 mol of ethyl thioxamate were added. Sodium hydroxide was added to adjust the reaction pH to 7.1. The reaction temperature was maintained at 10-12°C. Reaction 115min. The reaction solution was separated from the immobilized cefodizime synthase. The obtained product was adjusted to pH 2.9 with 15% hydrochloric acid, filtered, washed, and dried to obtain crude cefodizime acid. Dissolve the crude product of cefodizime in a mixed solvent of 95mL ethanol and 95mL acetone, dissolve 18g of sodium isooctyl alcohol in a mixed solvent of 36mL ethanol and 36mL acetone, slowly drop it into the above mixed solvent, and stir slowly until cloudy After crystallization, add 100 mL of toluene dropwise, grow the crystal at 10°C for 1 hour, filter with suction, wash with 20 mL of toluene, and dry in vacuo to obtain cefodizime sodium. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com