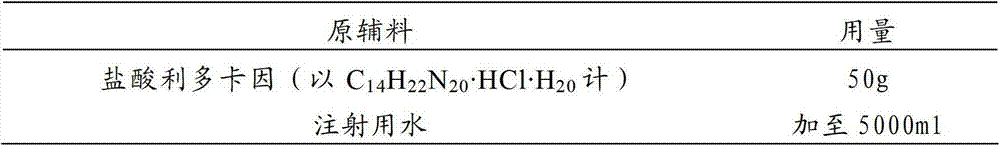Pharmaceutical composition of injection cefodizime sodium and lidocaine hydrochloride injection
A technology of lidocaine hydrochloride and cefodizime sodium, applied in the field of medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] The preparation of embodiment 1 injection cefodizime sodium
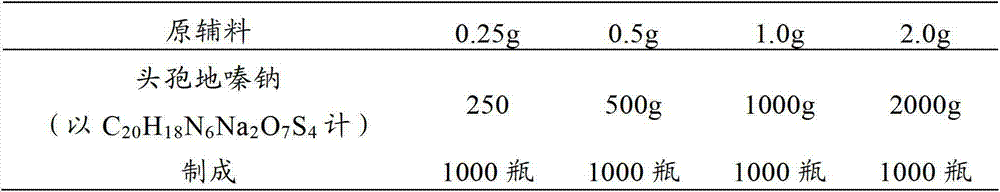
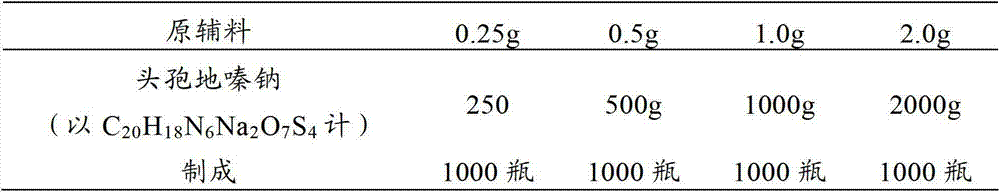
[0030] prescription:
[0031]
[0032] making process:
[0033] (1) 250g of cefodizime sodium sterile powder (in C 20 h 18 N 6 Na 2 o 7 S 4 meter) into the subpackaging room (the ambient temperature of the subpackaging post is 18°C-26°C, and the relative humidity is below 65%), debug the subpackaging machine, adjust the number of steps to adjust the loading volume, so that the loading volume reaches the specified range, and then distribute Put it in a low borosilicate glass control injection bottle, press the stopper completely, and crimp the cap.
[0034] (2) cefodizime sodium sterile powder 500g (in C 20 h 18 N 6 Na 2 o 7 S 4 meter) into the subpackaging room (the ambient temperature of the subpackaging post is 18°C-26°C, and the relative humidity is below 65%), debug the subpackaging machine, adjust the number of steps to adjust the loading volume, so that the loading volume reaches the spe...
Embodiment 2
[0037] Example 2 Preparation of Lidocaine Hydrochloride Injection
[0038] prescription:
[0039]
[0040] making process:
[0041] (1) Add a total of 4000ml of water for injection into the liquid mixing tank;
[0042] (2) Add 50g of lidocaine hydrochloride while stirring, and stir to dissolve completely;
[0043] (3) Weigh 2.5g of activated carbon for injection in the carbon mixing cabinet, add 5125ml of water for injection to wet the activated carbon for injection, and set aside;
[0044] (4) Add the wetted activated carbon for injection and stir for 30 minutes;
[0045] (5) The solution is decarburized by coarse filtration and filtered through a 0.45 μm cartridge filter;
[0046] (6) Add water for injection to 5000ml;
[0047] (7) Adjust the pH value of the liquid to 3.5-5.5.
[0048] (8) Sterilize and filter with a 0.22 μm microporous membrane until the visible foreign matter is qualified, fill with 2ml / bottle or 5ml / bottle, sterilize, seal, and get ready.
Embodiment 3
[0049] The preparation of embodiment 3 combination packaging medicines
[0050] Combination 1: Cefodizime Sodium for Injection 0.25g and Lidocaine Hydrochloride Injection 2ml.
[0051] Combination 2: Cefodizime Sodium for Injection 0.5g and Lidocaine Hydrochloride Injection 2ml.
[0052] Combination 3: Cefodizime Sodium for Injection 1.0g and Lidocaine Hydrochloride Injection 2ml.
[0053] Combination 4: Cefodizime Sodium for Injection 2.0g and Lidocaine Hydrochloride Injection 2ml.
[0054] Combination 5: Cefodizime Sodium for Injection 0.25g and Lidocaine Hydrochloride Injection 5ml.
[0055] Combination 6: Cefodizime Sodium for Injection 0.5g and Lidocaine Hydrochloride Injection 5ml.
[0056] Combination 7: Cefodizime Sodium for Injection 1.0g and Lidocaine Hydrochloride Injection 5ml.
[0057] Combination 8: Cefodizime Sodium for Injection 2.0g and Lidocaine Hydrochloride Injection 5ml.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com