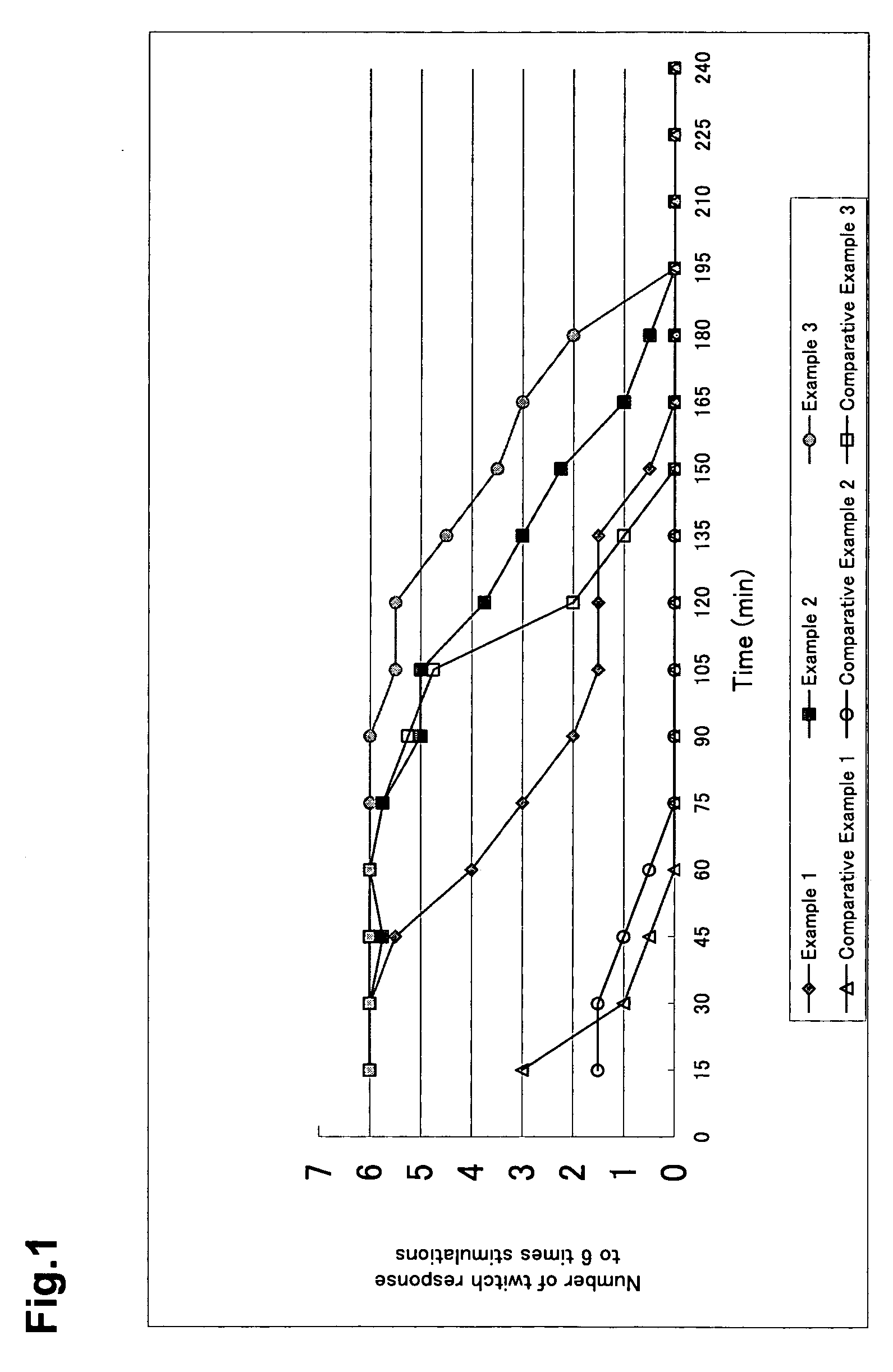
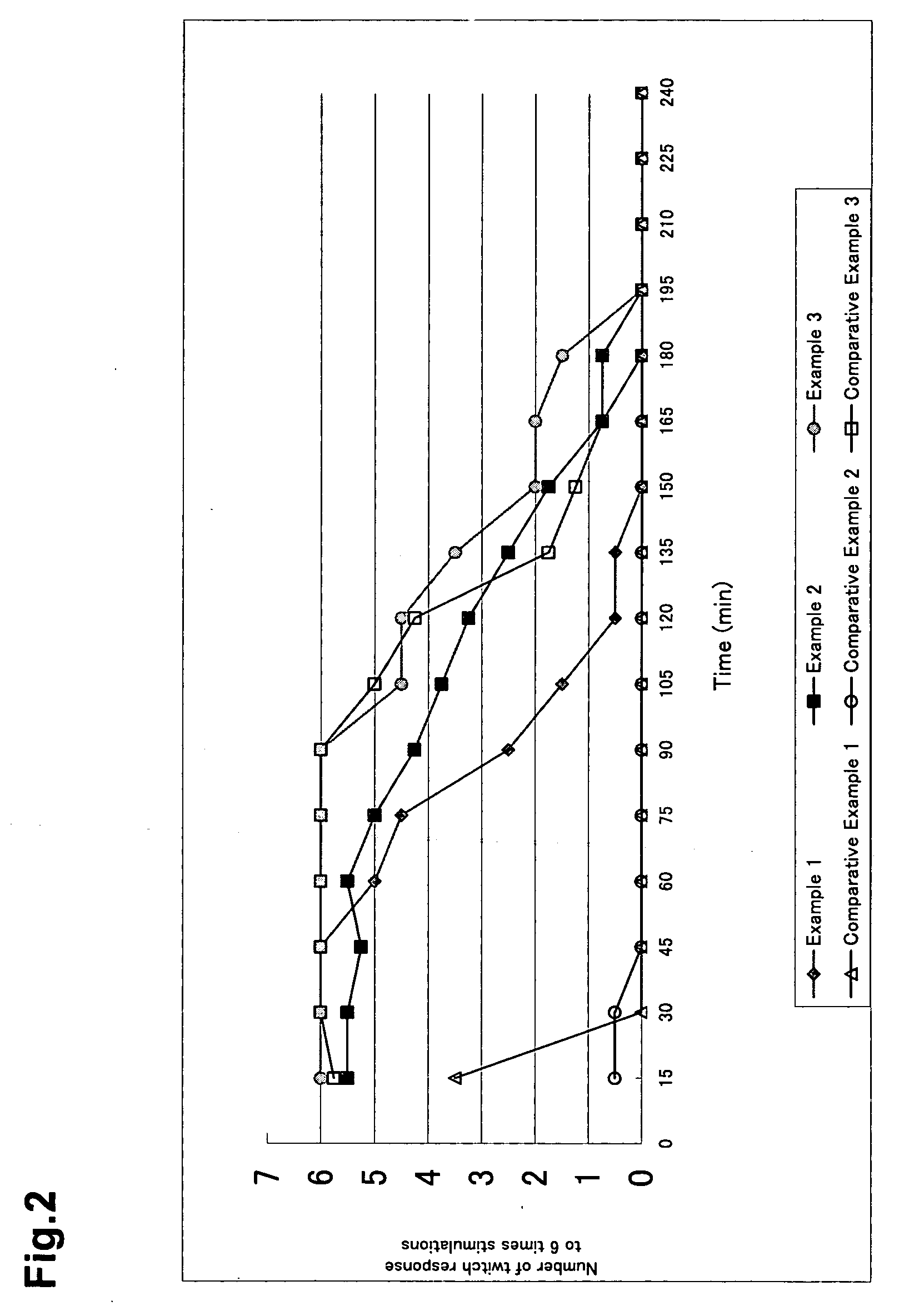
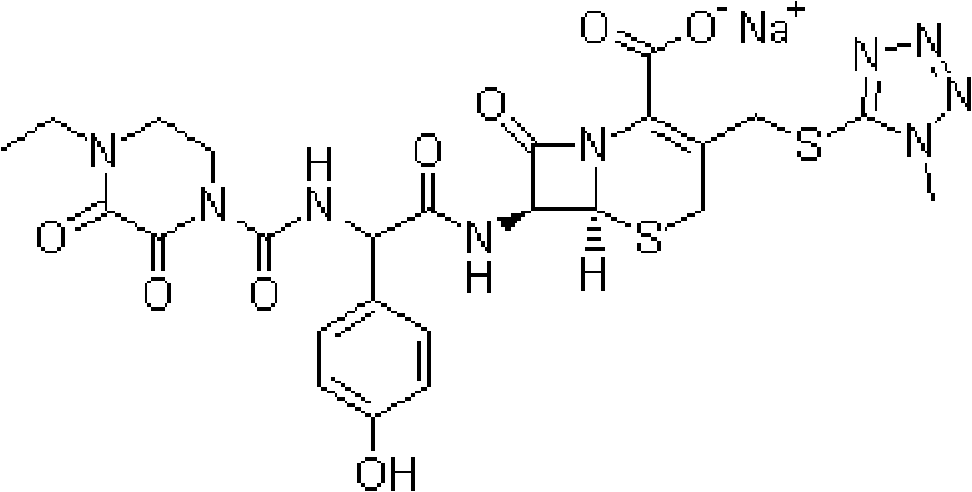
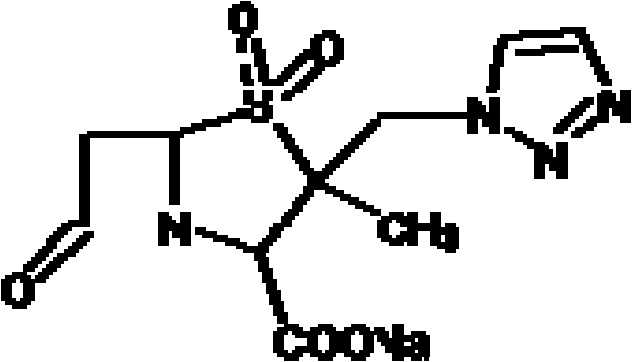
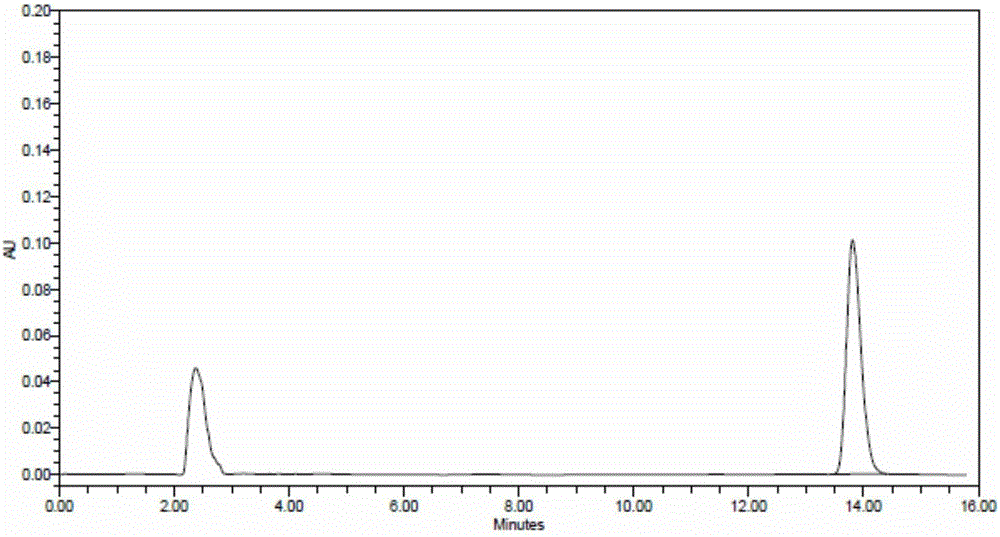
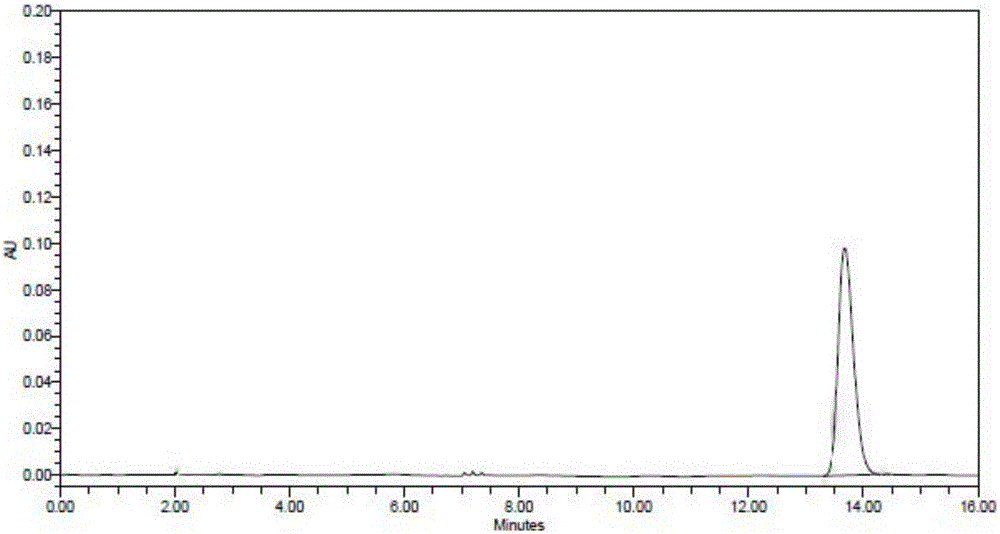
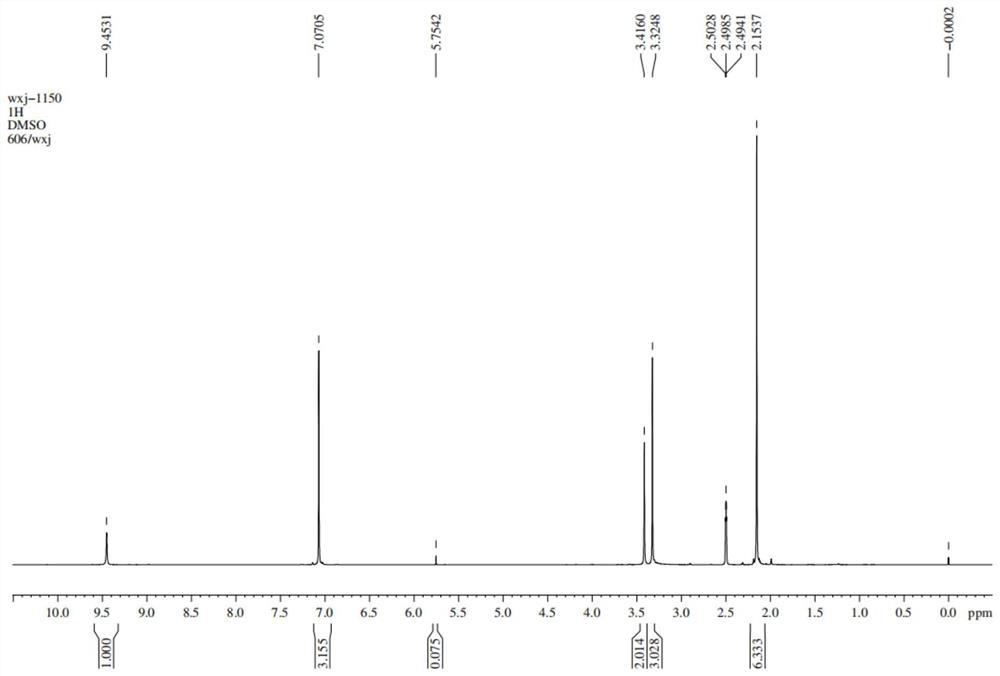
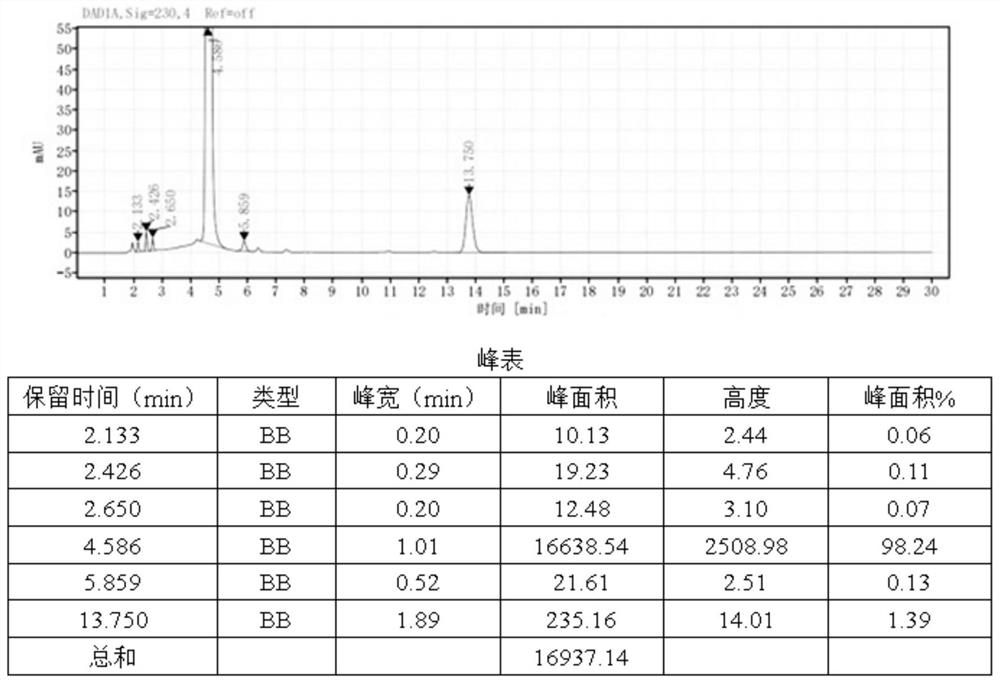
Patents
Literature
150 results about "Lidocaine Hydrochloride" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
The hydrochloride salt from of lidocaine, an aminoethylamide and a prototypical member of the amide class anesthetics. Lidocaine interacts with voltage-gated Na+ channels in the nerve cell membrane and blocks the transient increase in permeability of excitable membranes to Na+. This prevents the generation and conduction of nerve impulses and produces a reversible loss of sensation. Lidocaine hydrochloride also exhibits class IB antiarrhythmic effects. The agent decreases the flow of sodium ions into myocardial tissue, especially on the Purkinje network, during phase 0 of the action potential, thereby decreasing depolarization, automaticity and excitability.
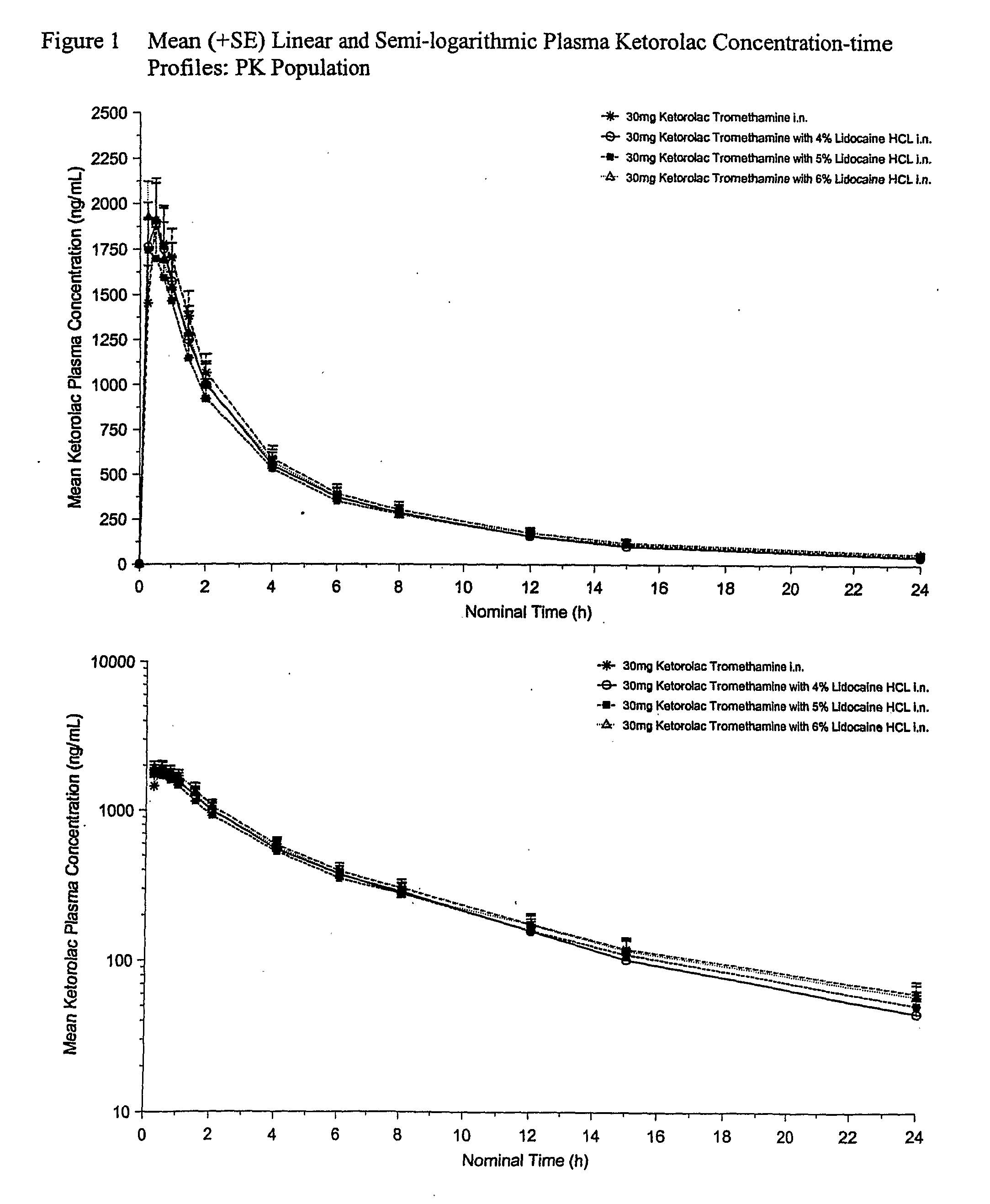
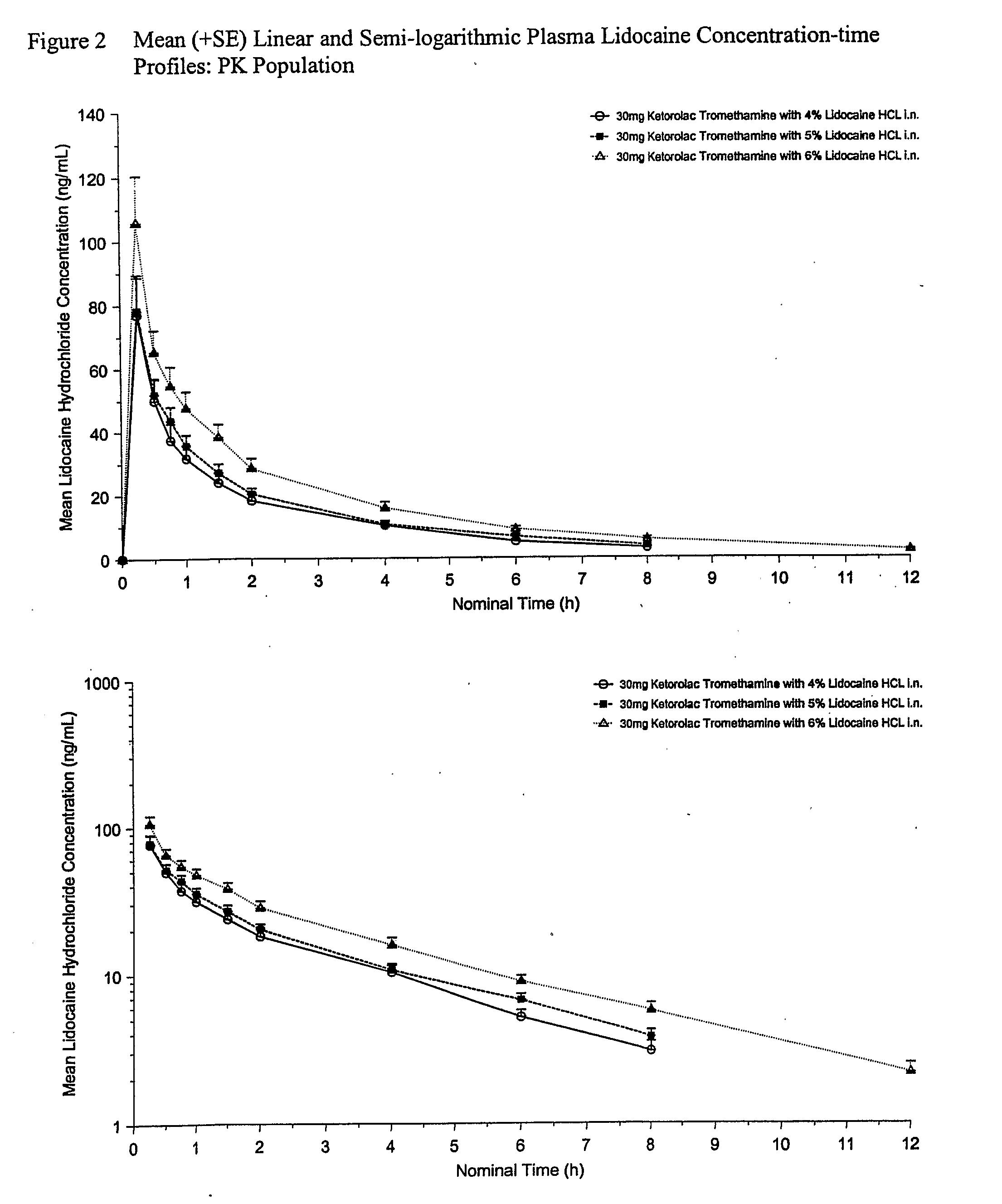
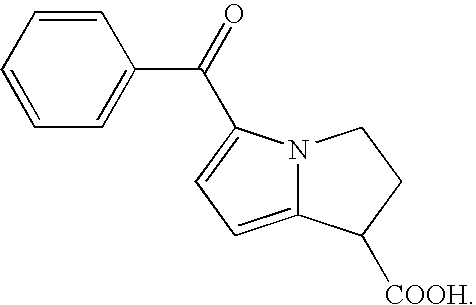
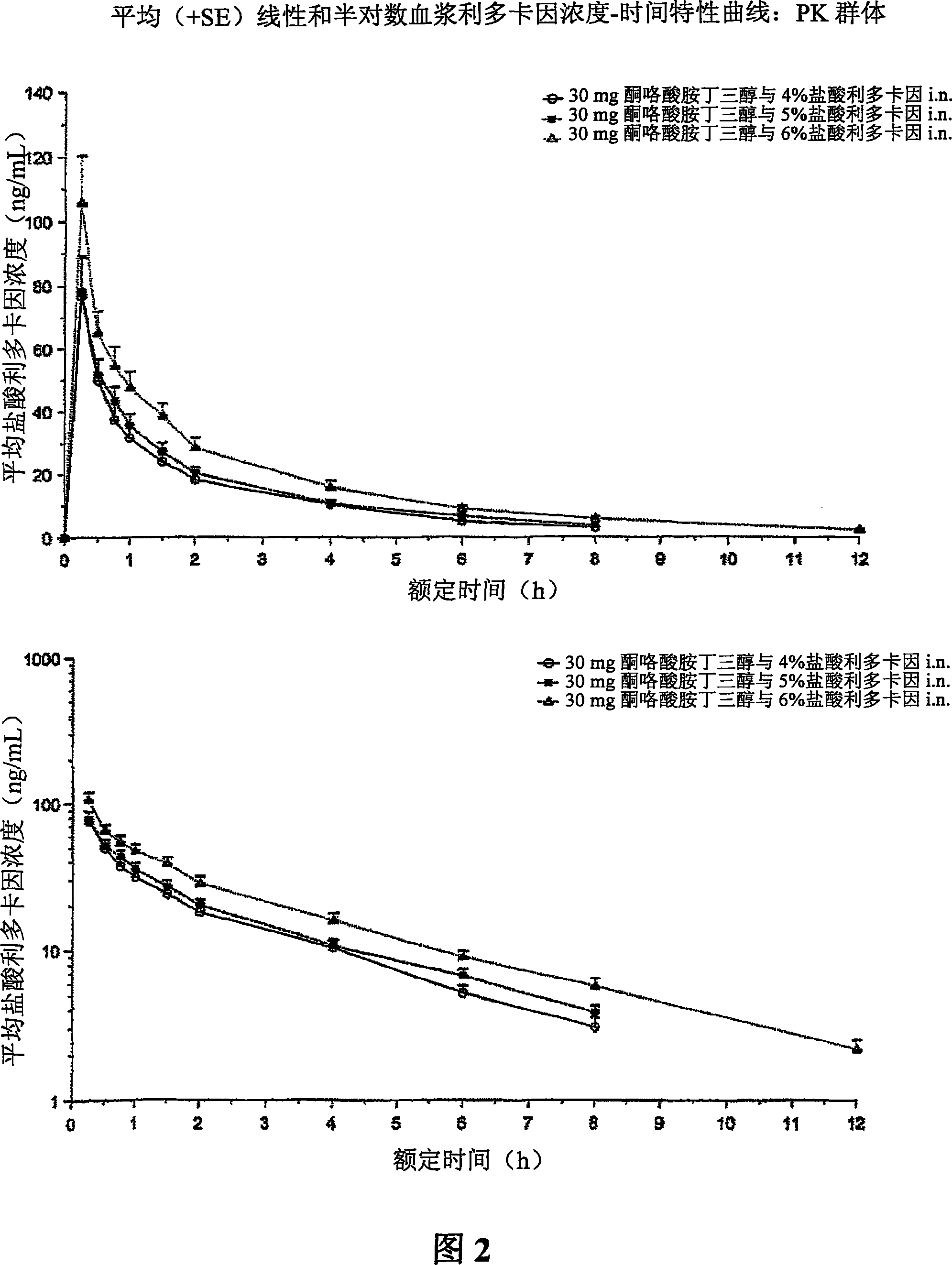
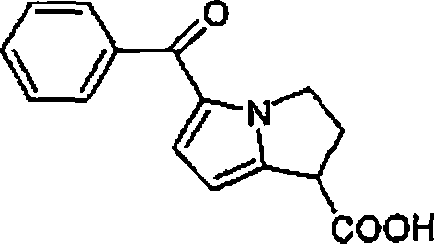
Therapeutic compositions for intranasal administration of ketorolac
Therapeutic compositions, particularly sprayable aqueous compositions, comprise ketorolac or a pharmaceutically acceptable salt, in combination with a local anesthetic, such as lidocaine hydrochloride. The compositions are nasally administered to a subject in need thereof to treat pain or inflammation and have the benefit of reduced stinging and improved efficacy, compared to known nasally administered compositions.
Owner:ROXRO PHARMA INC
Method of inducing topical anesthesia and transdermal patch
InactiveUS20090123527A1Avoid the needLower potentialPowder deliveryBiocideTransdermal patchPhysiological fluid
Disclosed is a method of inducing topical anesthesia in a tissue or organ of an animal comprising providing an aqueous gel formulation comprising water, an anesthetic (e.g., lidocaine hydrochloride), a viscoelastic polymer, and a tonicity modifier, wherein the aqueous gel formulation is free of preservatives and phosphate buffer, is isotonic with physiological fluids, and is sterile and has low particulate count. Also disclosed are a transdermal patch comprising the aqueous gel formulation suitable for applying on the skin of a patient and a method of controlling pain therewith.
Owner:AKORN
Aqueous gel formulation and method for inducing topical anesthesia
Disclosed is a stable aqueous gel formulation suitable for topical use comprising water, an anesthetic (e.g., lidocaine hydrochloride), a viscoelastic polymer, and a tonicity modifier, wherein the aqueous gel formulation is free of preservatives and phosphate buffer, is isotonic with physiological fluids, and is sterile and has low particulate count. Also disclosed is a method of inducing topical anesthesia on a tissue or organ, e.g., the eye, of an animal comprising providing a stable aqueous gel formulation comprising water, an anesthetic, a viscoelastic polymer, and a tonicity modifier, wherein the aqueous gel formulation is free of preservatives and phosphate buffer, is isotonic with physiological fluids, and is sterile, and topically administering an effective amount of the aqueous gel formulation to the tissue or organ of the animal.
Owner:AKORN
Aqueous gel formulation and method for inducing topical anesthesia
InactiveUS20080020044A1Avoid the needLower potentialPowder deliveryOrganic active ingredientsParticulatesPhysiological fluid
Disclosed is a stable aqueous gel formulation suitable for topical use comprising water, an anesthetic (e.g., lidocaine hydrochloride), a viscoelastic polymer, and a tonicity modifier, wherein the aqueous gel formulation is free of preservatives and phosphate buffer, is isotonic with physiological fluids, and is sterile and has low particulate count. Also disclosed is a method of inducing topical anesthesia on a tissue or organ, e.g., the eye, of an animal comprising providing a stable aqueous gel formulation comprising water, an anesthetic, a viscoelastic polymer, and a tonicity modifier, wherein the aqueous gel formulation is free of preservatives and phosphate buffer, is isotonic with physiological fluids, and is sterile, and topically administering an effective amount of the aqueous gel formulation to the tissue or organ of the animal.
Owner:AKORN
Compound analgesic agent
InactiveCN1679520ARelieve symptoms of acute and chronic painAntiviralNervous disorderAntipyreticCalcium hydroxideCarboxymethyl cellulose
A compound analgesic for bone fracture, injury, ecchymoma, nerval pain, etc is composed of antisticking layer, hydrophilic gel layer and substrate layer. Said hydrophilic gel layer consists of the medicine prepared from lidocaine hydrochloride and superfine notoginseng powder or its extract and the matrix prepared from gelatin, carboxymethyl cellulose sodium, polyvinyl pyrrolidone, etc.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
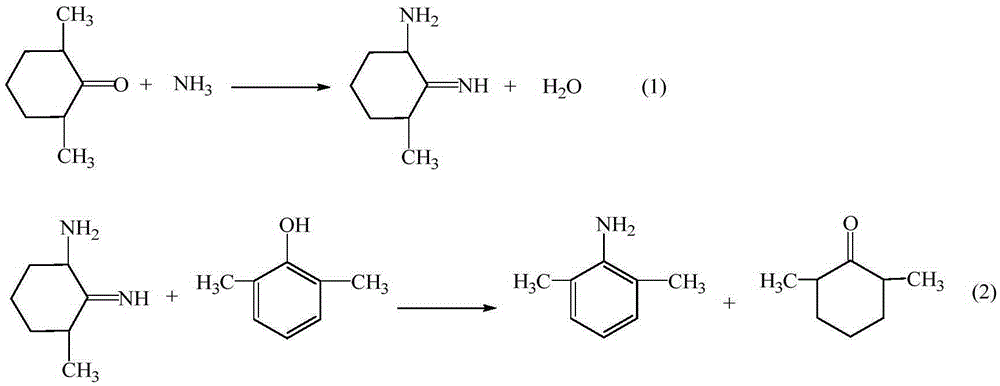
Method for preparing lidocaine hydrochloride
ActiveCN105294477AThe synthesis process is simpleHigh purityOrganic compound preparationCarboxylic acid amides preparationDimethylaniline N-oxideOrganic layer
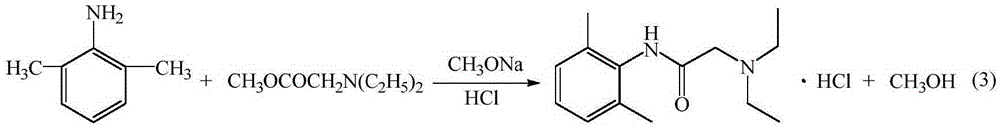
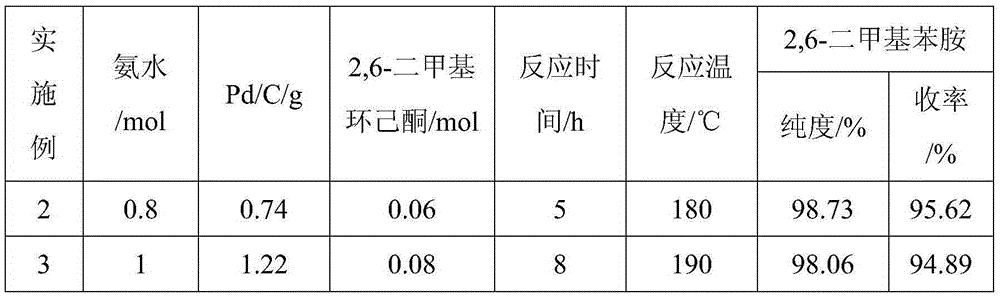
The invention provides a method for preparing lidocaine hydrochloride, and belongs to the technical field of anesthetic synthesis. The method comprises the following steps: by taking 2,6-xylenol as a raw material, Pd / C as a main catalyst and 2,6-dimethylcyclohexanone as a promoter, performing liquid phase amination with ammonia water at high temperature, thereby obtaining a midbody 2,6-dimethylaniline; enabling sodium methylate, 2,6-dimethylaniline and N,N-lignocaine methyl acetate as raw materials to react at 90-95 DEGC, distilling while reaction is performed to remove methanol till no methanol can be evaporated out, continuously reacting for 30 minutes, cooling to the room temperature, adding dichloroethane, washing with water, and leaving to stand to layer, thereby obtaining an organic layer, namely, a lidocaine based dichloroethane solution; further adding hydrochloric acid into the lidocaine based dichloroethane solution, adjusting the pH value to be 3.5-4 by using hydrogen chloride, adding activated carbon to reflux for 20-40 minutes, filtering, concentrating the filtrate, cooling, crystallizing, and dying, thereby obtaining lidocaine hydrochloride. The lidocaine hydrochloride prepared by using the method is simple in synthesis process and high in product purity, that is, the purity can be greater than 99%, and the total yield is greater than 84%.
Owner:ZHEJIANG ESIGMA BIOTECH CO LTD
Gargle for relieving pain for oral inflammation disease
InactiveCN101559077ANot pollutedGuaranteed chemical stabilityInorganic boron active ingredientsHydroxy compound active ingredientsDiseaseSodium bicarbonate
The invention belongs to the technical field of medicine, in particular to a drug composition of gargle which contains sodium dichlorophenolate and can relieve pain and be anti-inflammatory for the oral inflammation disease; wherein the drug composition comprises the following components by percentage: 0.10 to 30 percent of sodium dichlorophenolate hydroxypropyl-Beta-cyclodextrin inclusion (equal to 0.065 to 0.20 percent of concentration of sodium dichlorophenolate), 0.01 to 0.10 percent of lidocaine hydrochloride, 0.10 to 5.0 percent of borax, 0.1 to 2.0 percent of boric acid, 0.1 to 10 percent of glycerin, 0.10 to 5.0 percent of sodium bicarbonate, 0.001 to 0.10 percent of vitamin B12, 0.02 to 1.0 percent of tromethamine, 0.01 to 5.0 percent of sucralose, 0.10 to 5.0 percent of peppermint essence and 0.01 to 1.0 percent of sodium benzoate; a proper amount of pharmaceutical caramel color and water for injection is added till to reach the needed weight / volume concentration. All the above components are weighted by weight / volume percentage; the aqueous solution of the composition has the pH value of 6.5 to 9.0. The composition has little thrill to oral mucosa, stable storage for long time and good biological tolerance and has fast and long-lasting curative effect for relieving pain and being anti-inflammatory to the oral inflammation disease.
Owner:官培龙 +1
Aqueous gel formulation and method for inducing topical anesthesia
Disclosed is a stable aqueous gel formulation suitable for topical use comprising water, an anesthetic (e.g., lidocaine hydrochloride), a viscoelastic polymer, and a tonicity modifier, wherein the aqueous gel formulation is free of preservatives and phosphate buffer, is isotonic with physiological fluids, and is sterile and has low particulate count. Also disclosed is a method of inducing topical anesthesia on a tissue or organ, e.g., the eye, of an animal comprising providing a stable aqueous gel formulation comprising water, an anesthetic, a viscoelastic polymer, and a tonicity modifier, wherein the aqueous gel formulation is free of preservatives and phosphate buffer, is isotonic with physiological fluids, and is sterile, and topically administering an effective amount of the aqueous gel formulation to the tissue or organ of the animal.
Owner:AKORN
Gel for treating herpes zoster and preparation method thereof
The invention discloses gel for treating herpes zoster. The gel is prepared from the following raw materials in percentage by weight: 2-4 percent of acyclovir, 1-3 percent of lidocaine hydrochloride, 0.05-0.15 percent of tacrolimus, 0.04-0.06 percent of vitamin B12, 0.5-1.5 percent of a gel substrate, 1-1.5 percent of a neutralizer, 4-6 percent of a moisturizer, 25-35 percent of a solvent, 1-3 percent of a solubilizer, 0.5-1.5 percent of an absorption enhancer and the balance of water. The gel substrate is any one or more of carbopol 940, hydroxypropyl methyl cellulose, alginate, gelatin or starch; the neutralizer is triethanolamine or sodium hydroxide; the moisturizer is glycerin; the solvent is ethyl alcohol; the solubilizer is tween-80; the absorption enhancer is laurocapram. The invention also discloses a preparation method of the gel. All medicine components in the gel have a synergistic effect, the dosage of the medicines can be reduced, and the adverse reaction of the medicines is reduced.
Owner:济南众志康成药物研发有限公司
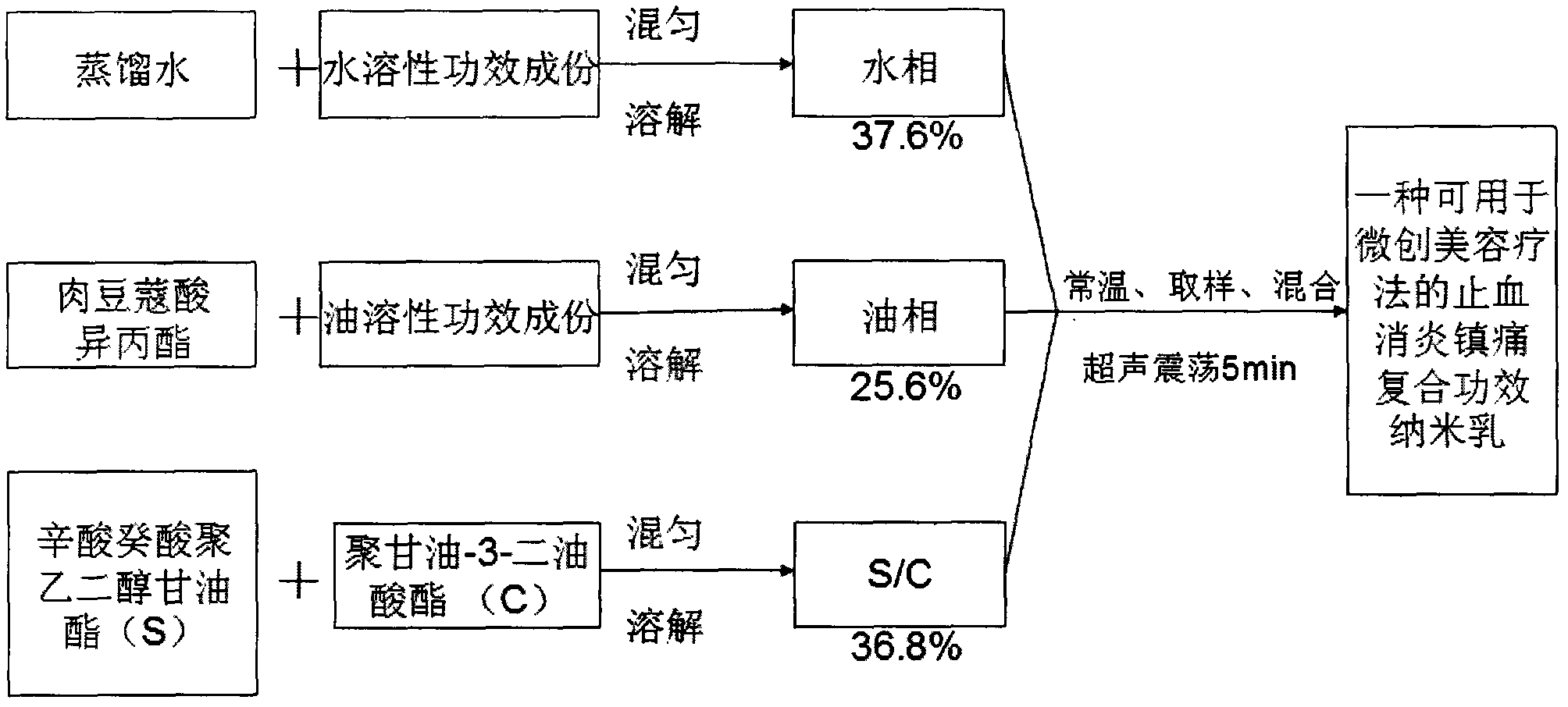
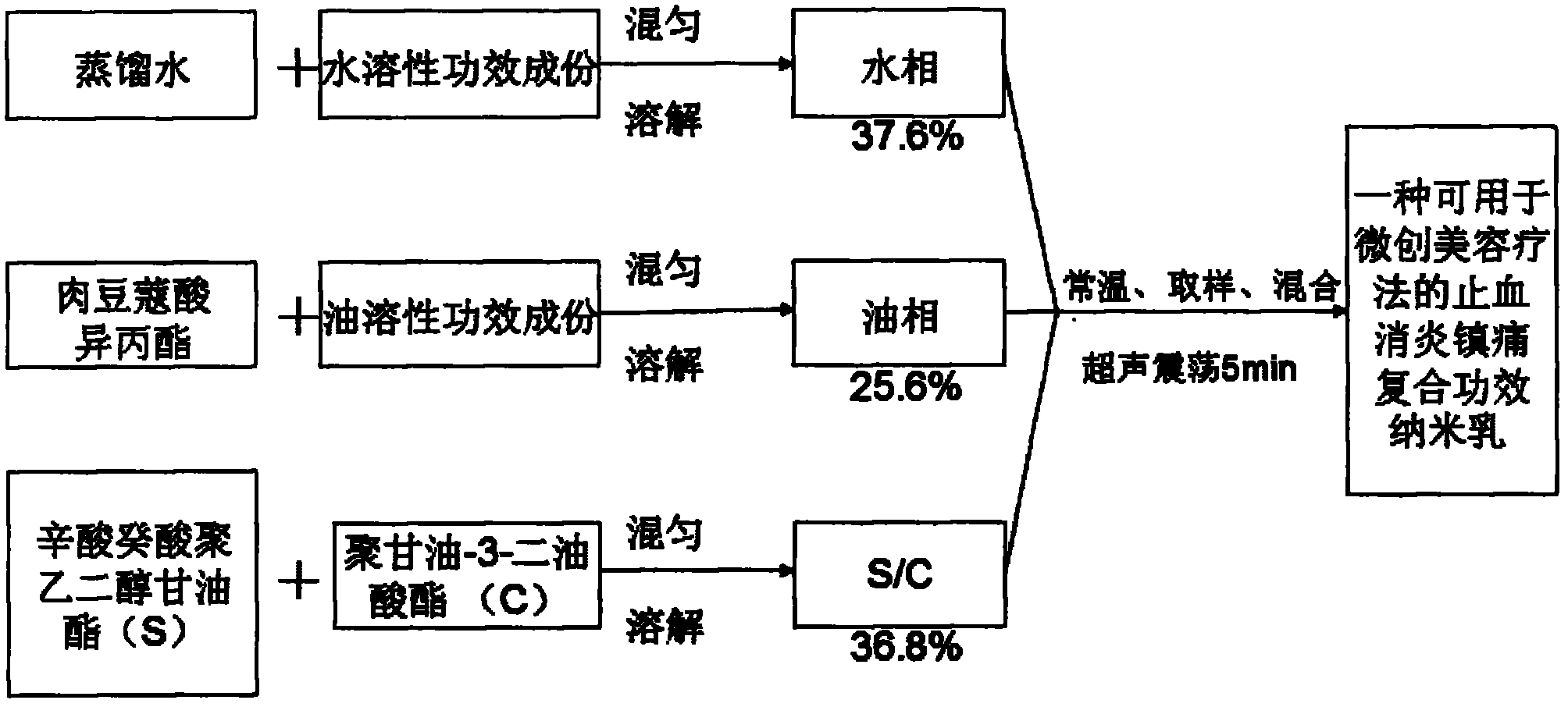
A hemostatic, anti-inflammatory and analgesic composite effect nanoemulsion that can be used for minimally invasive cosmetic therapy and its preparation method
ActiveCN102274493AImprove hemostatic efficiencyVersatileOrganic active ingredientsPeptide/protein ingredientsSide effectFreeze-drying
The invention relates to bleeding-stopping, inflammation-diminishing and pain-relieving nano emulsion for use in minimally invasive beauty treatment therapy and a preparation method thereof. The nano emulsion is prepared from the following raw materials in part by weight: 0.0055 part of thrombase freeze-dried powder, 0.0005 part of epinephrine, 5 parts of calcium chloride, 1 part of sodium chloride, 6 parts of coix seed essence oil, 6 parts of as arum volatile oil, 5 parts of lidocaine hydrochloride, 13.6 parts of isopropyl myristate, 27.6 parts of octoic acid and capric acid polyethylene glycol glyceride, 9.2 parts of polyglycerol-3-dioleate and 26.594 parts of distilled water. The nano emulsion has diversified functions and has bleeding-stopping, inflammation-diminishing and pain-relieving effects, so the complex process of the minimally invasive beauty treatment therapy is simplified. In addition, a nano transdermal technique is adopted to promote the functional components to enterskin effectively, constantly and controllably to produce the bleeding-stopping, inflammation-diminishing and pain-relieving effects; and the toxic and side effects of the nano emulsion can be reducedgreatly.
Owner:董萍 +1
Composition for local anesthesia
InactiveUS20060216245A1Excellent durability of actionDurability of actionBiocideCosmetic preparationsCatecholamineHydroxyzine Hydrochloride
A composition for local anesthesia which comprises a local anesthetic such as lidocaine hydrochloride as an active ingredient and an agent for sustaining anesthetic action selected from the group consisting of antihistamines such as diphenhydramine hydrochloride or hydroxyzine hydrochloride, and does not substantially contain catecholamines such as epinephrine, which has increased durability of anesthetic action without using catecholamines, and is useful as a safe composition for local anesthesia for performing short-time dental operations such as tooth extraction or oral surgery operations.
Owner:SHOWA YAKUHIN KAKO
Pharmaceutical composition for easing pain and use thereof
The invention relates to a pharmaceutical composition for easing pain. The pharmaceutical composition is prepared from the following components according to the volume ratio: 2% methylthioninium chloride injection, 0.5% bupivacaine hydrochloride injection, 2% lidocaine hydrochloride injection and water for injection, of which the ratio is 2:5:5:16. The pharmaceutical composition relieves pain after being applied to an anorectal postoperative incision, improves local blood circulation, benefits for wound concrescence, contributes to wound bleeding, and reduces secreta. The pharmaceutical composition does not generate systemic adverse reaction after an operation, local edema or necrosis by a clinical test, and has no influence on local granulation tissue growth and wound concrescence; the anus has no abnormity, can smoothly defecate after the wound is repaired, has no untoward effect after follow-up treatment, and is reliable in curative effect. The drug disclosed by the invention removes a local hot pain after methylenum coeruleum is injected, does not generate an anesthesia clearance, is fast to take effect, and takes effect after being injected 2-4 hours; the reversible nerve damage disappears after about 15 days in general; and the requirement of wound growth just can be met.
Owner:临江市妇幼保健院
Injection powder and injection preparation of cefoperazone sodium-tazobactam combination
ActiveCN102552275AImprove efficacyAntibacterial agentsPowder deliveryCurative effectInjection powder
The invention relates to the technical field of medicine, in particular to an injection powder and injection preparation of a cefoperazone sodium-tazobactam combination. The injection powder and injection preparation comprises cefoperazone sodium, tazobactam combination and lignocaine hydrochloride, wherein the mass ratio of the cefoperazone sodium to the tazobactam combination to the lignocaine hydrochloride is 4:1:(0.01-0.05). Compared with a positive medicament control group, the injection powder and injection preparation of the cefoperazone sodium-tazobactam combination provided by the invention has the advantages of capability of relieving injection pain and remarkable improvement on the curative effect.
Owner:SHANDONG LUOXIN PARMACEUTICAL GROUP STOCK CO LTD +2
Percutaneous absorption film coating agent for treating rheumatoid arthritis and preparation method thereof
InactiveCN101991585AEasy to useNo oralOrganic active ingredientsAntipyreticSolventEarly rheumatoid arthritis
Owner:FUJIAN ACAD OF MEDICAL SCI
Lidocaine hydrochloride mucilage preparation method
InactiveCN101385702AOpen alternatelyEasy to closeOrganic active ingredientsAerosol deliveryDrug contentGlycerol
A method for preparing a lidocaine hydrochloride mucilage comprises the following steps: a formula amount of ethyl p-hydroxybenzoate is put into purified water which accounts for 25% of the total volume of the ethyl p-hydroxybenzoate, heated and stirred until complete dissolution, added with a formula amount of lidocaine hydrochloride and stirred for dissolution, added with a formula amount of glycerol and stirred evenly; additionally, a formula amount of sodium carboxymethyl cellulose is added to purified water which accounts for 50% of the total volume of the sodium carboxymethyl cellulose, and stirred for complete swelling; the two solutions obtained are mixed evenly, and added with 0.1N sodium hydroxide to adjust the pH value to 6.0-7.0; the monarch drug content of the obtained mucilage is determined; the mucilage is added with water to sufficient amount, mixed evenly and filled, and sterilized by 110 DEG C saturated vapor for 30 minutes; inspected under a lamp and packaged to obtain the finished product. Compared with the prior art, the lidocaine hydrochloride mucilage has the advantages of matching anaesthesia and inspection time, good lubricating property and sterility assurance level.
Owner:浙江康德药业集团股份有限公司
Lidocaine hydrochloride polymeric liposome for topical anesthesia and preparation method
InactiveCN102406609AHigh encapsulation efficiencyImprove permeabilityOrganic active ingredientsAnaestheticsProbe typeRotary evaporator
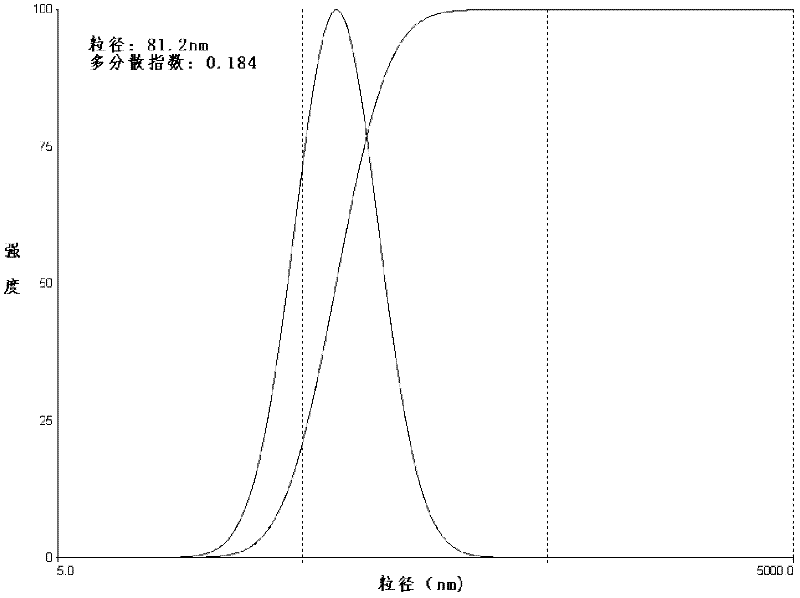
The invention relates to lidocaine hydrochloride polymeric liposome for topical anesthesia and a preparation method. The preparation method comprises the following steps: dissolving OQLCS (octadecyl-quaternized lysine modified chitosan) and cholesterol in dichloromethane to form an oil phase; completely dissolving lidocaine hydrochloride in deionized water to form a water phase; performing ultrasonic treatment to the oil phase in a power range of between 150 and 200W, adding the water phase, and performing ultrasonic dispersion by using a probe type ultrasonic generator until a semitransparent emulsion is formed to form a dispersion emulsion with uniform water and oil; and performing rotary evaporation to the emulsion on a rotary evaporator at 40 DEG C at a rotating speed of 50r / min, and introducing nitrogen flow into the rotary evaporator for protecting, and filtering through a sterile filter membrane of between 0.2 and 0.4mu m after organic solvent is completely volatilized to obtain the lidocaine hydrochloride polymeric liposome. The encapsulation rate of the liposome to a medicament is between 60 and 85 percent; the grain diameter of the polymeric liposome is between 60 and 180nm, and the surface is positively charged with an electric potential of between 6 and 65mv. The lidocaine hydrochloride polymeric liposome can be directly applied to the surface of skin mucous membrane, or is further prepared to form gel or ointment.
Owner:STOMATOLOGICAL HOSPITAL TIANJIN MEDICAL UNIV
Anti-rheumatoid arthritis drug gel containing paclitaxel liposome and preparation method of gel
InactiveCN104688721AQuick Pain ReliefImprove bioavailabilityOrganic active ingredientsAntipyreticTreatment effectBioavailability
The invention relates to anti-rheumatoid arthritis drug gel containing paclitaxel liposome and a preparation method of the gel. The gel is prepared from main drugs and blank gel, wherein the main drugs comprise paclitaxel liposome and lidocaine hydrochloride; the mass volume ratio of the paclitaxel liposome to lidocaine hydrochloride is 5:100-20:100; and the mass volume ratio of lidocaine hydrochloride to the blank gel is 1:100-5:100. According to the requirements of evidence-based medicine, a drug paclitaxel for treating RA and lidocaine are compatible with each other, the paclitaxel is coated by utilizing a liposome technology, the bioavailability is improved, and the prepared gel suitable for directed use at an affected part is adopted. The gel achieves a treatment effect aiming at the pathogenesis of RA, and pain of a patient suffering from RA can be rapidly relieved. A novel therapy approach is provided for vast patients suffering from RA.
Owner:黄萍
Therapeutic compositions for intranasal administration of ketorolac
Therapeutic compositions, particularly sprayable aqueous compositions, comprise ketorolac or a pharmaceutically acceptable salt, in combination with a local anesthetic, such as lidocaine hydrochloride. The compositions are nasally administered to a subject in need thereof to treat pain or inflammation and have the benefit of reduced stinging and improved efficacy, compared to known nasally administered compositions.
Owner:ROXRO PHARMA INC
Bleeding-stopping, inflammation-diminishing and pain-relieving nano emulsion for use in minimally invasive beauty treatment therapy and preparation method thereof
ActiveCN102274493BImprove hemostatic efficiencyVersatileOrganic active ingredientsPeptide/protein ingredientsSide effectFreeze-drying
The invention relates to bleeding-stopping, inflammation-diminishing and pain-relieving nano emulsion for use in minimally invasive beauty treatment therapy and a preparation method thereof. The nano emulsion is prepared from the following raw materials in part by weight: 0.0055 part of thrombase freeze-dried powder, 0.0005 part of epinephrine, 5 parts of calcium chloride, 1 part of sodium chloride, 6 parts of coix seed essence oil, 6 parts of as arum volatile oil, 5 parts of lidocaine hydrochloride, 13.6 parts of isopropyl myristate, 27.6 parts of octoic acid and capric acid polyethylene glycol glyceride, 9.2 parts of polyglycerol-3-dioleate and 26.594 parts of distilled water. The nano emulsion has diversified functions and has bleeding-stopping, inflammation-diminishing and pain-relieving effects, so the complex process of the minimally invasive beauty treatment therapy is simplified. In addition, a nano transdermal technique is adopted to promote the functional components to enterskin effectively, constantly and controllably to produce the bleeding-stopping, inflammation-diminishing and pain-relieving effects; and the toxic and side effects of the nano emulsion can be reducedgreatly.
Owner:董萍 +1
Percutaneous adhering preparation containing hydrochloride lignocaine and peppermintcamphor
A transdermal paster for treating acute or chronic local pain is prepared from lidocain hydrochloride and methol. It can be used for the ultrasonic-type, ion leading-in type, electric perforating type, or high-pressure gas type medicine supplying system.
Owner:陶燃
Percutaneous absorption biological patch for treating rheumatoid arthritis and preparing method thereof
InactiveCN102085199AEasy to useNo oralOrganic active ingredientsAntipyreticSolventEarly rheumatoid arthritis
The invention relates to a percutaneous absorption biological patch for treating rheumatoid arthritis and preparing method thereof. The biological patch consists of basic remedy treating for the rheumatoid arthritis, medicament solvent, adhesive, biological plaster matrix and percutaneous absorption penetration enhancer. The basic remedies treating for the rheumatoid arthritis include triptolide and analgesics lidocaine hydrochloride alleviating the pain of the rheumatoid arthritis. The biological plaster has comprehensive pharmacological effect, exactly blocks up the main pathology link of development of rheumatoid arthritis, has strong pertinency and lasting pharmacological effect; and can be prepared by using a general method in biological plaster preparing technology field; and the preparing method is simple.
Owner:FUJIAN ACAD OF MEDICAL SCI
Sunburn treatment and sunburn prevention method
InactiveUS20050025723A1Prevent sunburnReduce sunburn inflammationCosmetic preparationsOrganic active ingredientsUltravioletUltraviolet radiation
The present invention is directed to a treatment for sunburn and a method for preventing sunburn. One aspect of this invention involves a sunburn treatment comprising one or more polypeptides with an amino acid sequence including KPV (SEQ. ID. NO. 1), MEHFRWG (SEQ. ID. NO. 2), HFRWGKPV (SEQ. ID. NO. 3), or SYSMEHFRWGKPV (SEQ. ID. NO. 4) for the treatment of the cutaneous inflammation caused by exposure to ultraviolet radiation. The polypeptides are at a level to effectively treat the cutaneous inflammation and are carried by a carrier. The one or more polypeptides can also be a dimer formed from any of the amino acid sequence above. In one preferred embodiment of the invention, the one or more polypeptides are used to prevent sunburn. In another preferred embodiment, the one or more polypeptides are dissolved in a carrier. In another preferred embodiment, the carrier includes aloe vera and lidocaine hydrochloride. In another preferred embodiment of the invention, the one or more polypeptides are dissolved in a liquid that is associated with an absorbent material for application to sunburned skin.
Owner:LIPTON JAMES M
Chitosan bleeding stopping and pain relieving powder and preparation method thereof
The invention relates to chitosan bleeding stopping and pain relieving powder and a preparation method thereof. The chitosan bleeding stopping and pain relieving powder is prepared from the following components in parts by weight: 0.1 to 10 parts of carboxymethyl chitosan, 0.1 to 10 parts of sodium alginate, 0.0004 part of thrombin and 0.1 to 2 parts of lidocaine hydrochloride. The components are cross-linked by calcium chloride, washed, dried and ground into powder. The chitosan bleeding stopping and pain relieving powder and the preparation method thereof have the advantages that the preparation method is simple and green; the production cost is low; the water-absorption swelling rate is high, the convenience in use is realized, and the bleeding stopping and pain relieving effects are good.
Owner:HENAN TUOREN BEST MEDICAL DEVICE CO LTD
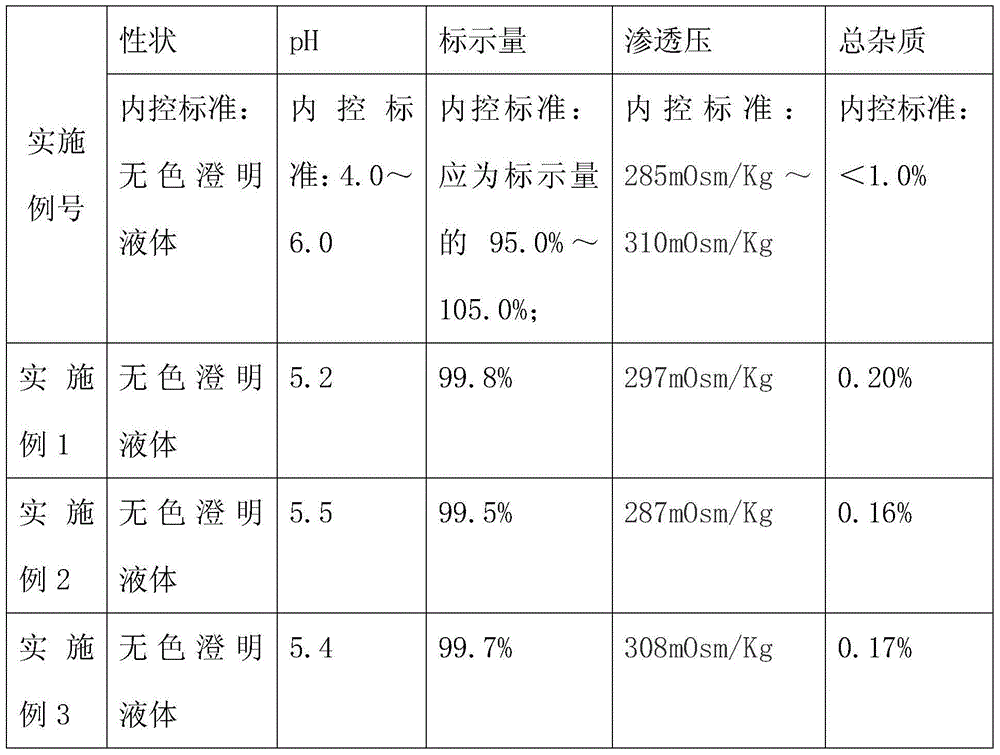
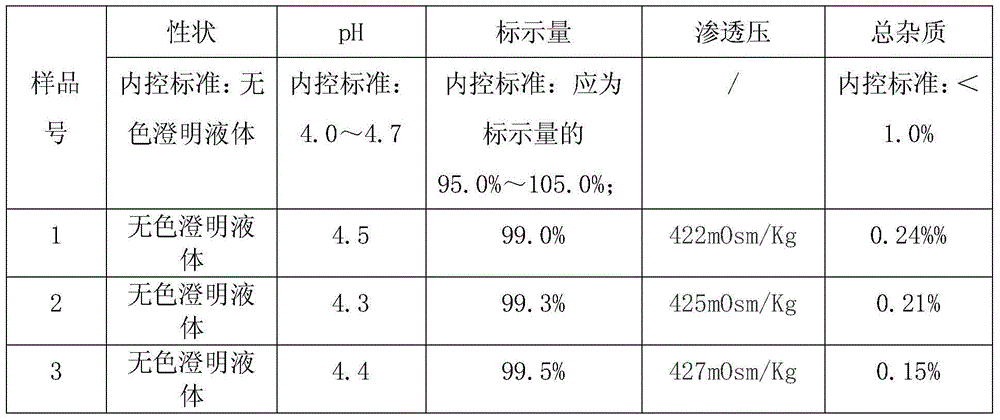
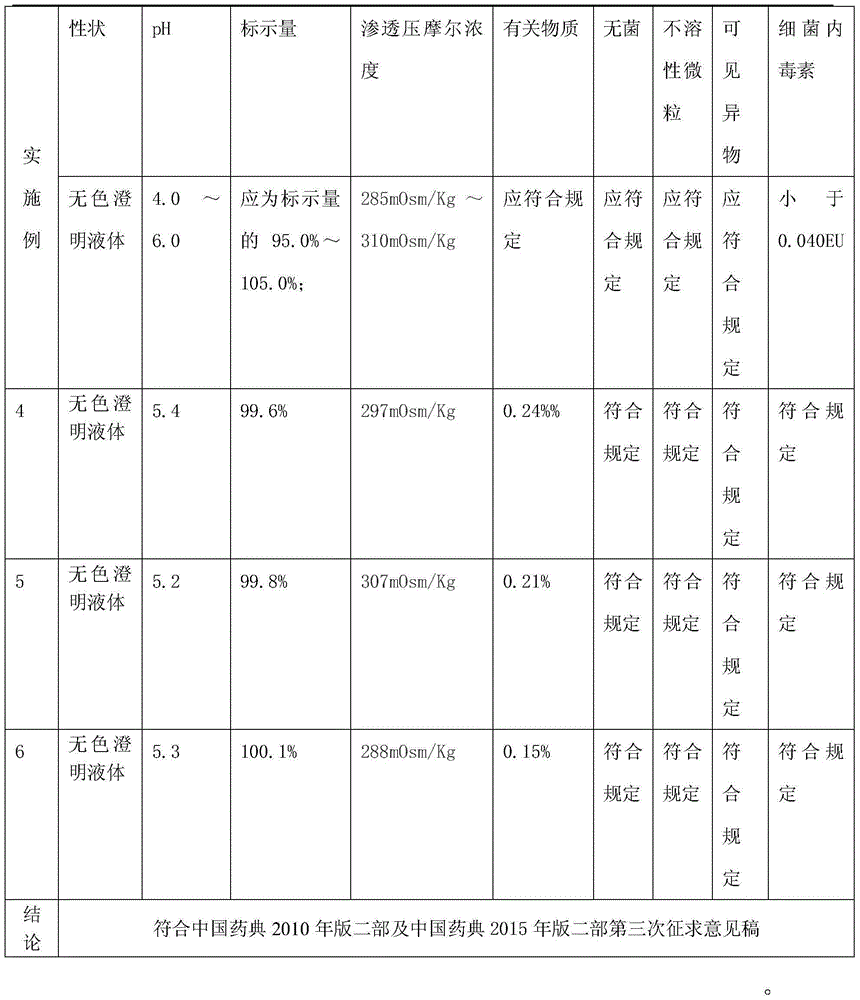
Lidocaine hydrochloride injection and preparation method thereof
InactiveCN105663035AConvenient for clinical operationReduce clinical adverse reactionsOrganic active ingredientsPharmaceutical delivery mechanismLarge applicationsPharmacology
The invention provides lidocaine hydrochloride injection. Each 1000ml of the lidocaine hydrochloride injection is prepared from the following components by weight: 20g of lidocaine hydrochloride (the theoretical weight, and the quantity of lidocaine hydrochloride needs to be determined according to the content as well as the water drying and the purifying condition), and 4.4-5.3 g of sodium chloride. According to the improved lidocaine hydrochloride injection and the preparation method of the lidocaine hydrochloride injection, the prescription composition is simple, and a few kinds of auxiliary materials is adopted, thus the clinical risks caused by lack of iso-osmia of a product are eradicated, and the various index including the pH of the product and related substances are stable and controllable, and meet the requirements in the exposure draft of the lidocaine hydrochloride injection in the China pharmacopeia 2015; the lidocaine hydrochloride injection is suitable for clinical application, and the product is safe and effective. A preparation method is simple, new cost is not increased, and the lidocaine hydrochloride injection is suitable for large-scale industrial production and has a large application value.
Owner:SHANGHAI ZHAOHUI PHARMA
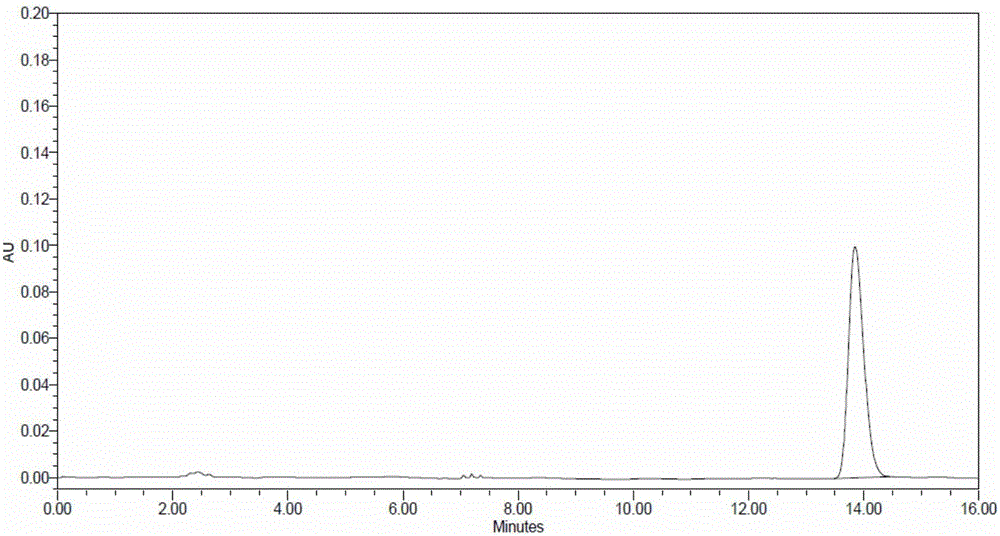
Method for detecting content of lidocaine hydrochloride in medical cross-linked sodium hyaluronate gel
InactiveCN106248819AImprove detection accuracyGood repeatabilityComponent separationCross-linkFiltration
The invention relates to a method for detecting lidocaine hydrochloride content in medical cross-linked sodium hyaluronate gel. The specific method is as follows: the product is hydrolyzed by hyaluronidase, filtered after completion, and the content is determined by high-performance liquid chromatography. The invention is for detecting the content of lidocaine hydrochloride in medical cross-linked sodium hyaluronate. The product is hydrolyzed by hyaluronidase, fluidity is added, mixed uniformly, filtered by a filter membrane, and then detected by a high-performance liquid chromatograph. The hydrolysis of the product reduces the influence of sodium hyaluronate on the detection, improves the detection accuracy, is easy to operate, and has good repeatability.
Owner:ZHEJIANG JINGJIA MEDICAL TECH CO LTD
Preparation method of lidocaine hydrochloride impurity E
ActiveCN111995539AHigh purityHigh yieldOrganic compound preparationCarboxylic acid amides preparationMorpholineAniline
The invention relates to a preparation method of a lidocaine hydrochloride impurity E, and belongs to the technical field of compound synthesis processes. The preparation method of the lidocaine hydrochloride impurity E comprises the following steps that: 4-morpholine-3-aniline is dissolved in a solvent; then an obtained solution reacts with substituted 2, 6-dimethylphenyl acetamide under the action of an acid-binding agent to generate the lidocaine hydrochloride impurity E. The lidocaine hydrochloride impurity E with higher purity can be obtained, the yield of the lidocaine hydrochloride impurity E is higher, and the purification steps are fewer.
Owner:ZHENGZHOU SIGMA CHEM
Nutritional injection for neck skin repair and application method thereof
InactiveCN111632070AProliferativeStrong tissue repairOrganic active ingredientsCosmetic preparationsAutologous tissueSkin repair
The invention discloses a nutritional injection for neck skin repair. Every 120 ml of the nutritional injection contains 95-110 ml of a sodium chloride solution, 5-10 ml of dimethylaminoethanol, 1-5 ml of cationic hyaluronic acid, 5-10 ml of a lidocaine hydrochloride injection and 110-120 million units of fibroblasts. By injection of a composite fiber nutrient solution, autologous tissues are self-healed and regenerated, and the effect is more natural. Meanwhile, collagen and elastin are continuously secreted, and the effect is more durable.
Owner:上海鄄飞健康管理咨询有限公司
DNA medicated collection kits
Provided are kits for collecting samples from a person for DNA (Deoxyribonucleic acid) testing. The kits can optionally also allow for collection of a urine sample. Components of the kits can include lid local anesthetic such as lidocaine hydrochloride, an item that allows for cleaning the mouth, such as mouthwash, a tool for removing DNA such as buccal swabsticks, a container for collecting urine, such as a urine cup, a pair of gloves, a face mask, and a CSR wrap. DNA samples are collected from the buccal area by scraping the area with a buccal swabstick provided in the kit.
Owner:ASCLEMED USA
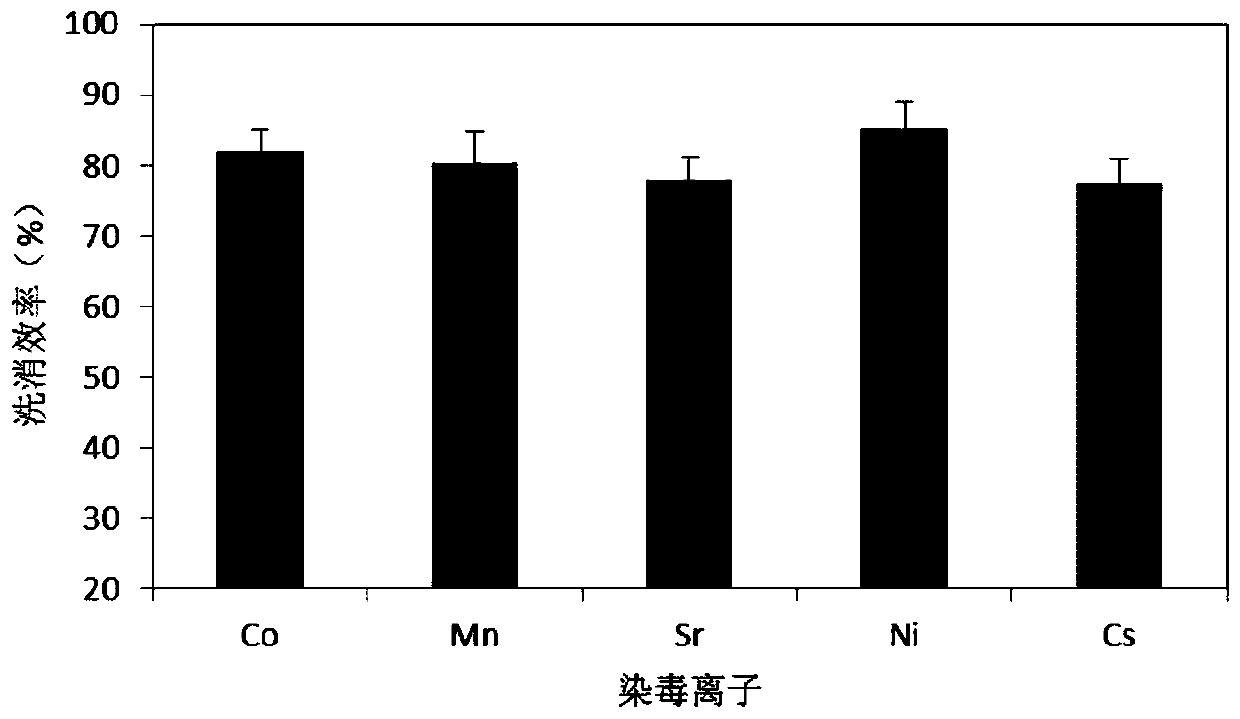
Radionuclide pollution decontamination agent and preparation method and application thereof
ActiveCN109700826AExtensive coordination abilityGood biocompatibilityOrganic active ingredientsAntinoxious agentsSide effectPhosphate
The invention relates to a radionuclide pollution decontamination agent and a preparation method and an application thereof. The decontaminant comprises the following components by mass percentage: 1.0%-20% of EDTA.Na2, 0.1%-0.4% of carboxymethyl chitosan, 0.05%-0.3% of sodium alginate, 0.5%-2.0% of lidocaine hydrochloride, and balance of pure water or phosphate buffer, wherein a pH value is 6.9-7.0. The preparation method comprises the following steps: EDTA-Na2, carboxymethyl chitosan, sodium alginate and lidocaine hydrochloride are accurately weighed according to the proportion, the EDTA-Na2is heated and dissolved under alkaline conditions, and then the carboxymethyl chitosan and sodium alginate are dissolved, and then the two are mixed and stirred uniformly, a lidocaine hydrochloride solution is added, the pH is adjusted to 6.7-6.9 with HCl, the material is dissolved while stirring, and metering a volume. The decontamination agent has a chelate adsorption function on a plurality ofradionuclides, has no toxic and side effects, and can be used as the low-toxic and high-efficiency radionuclide pollution decontamination agent.
Owner:中国人民解放军海军特色医学中心
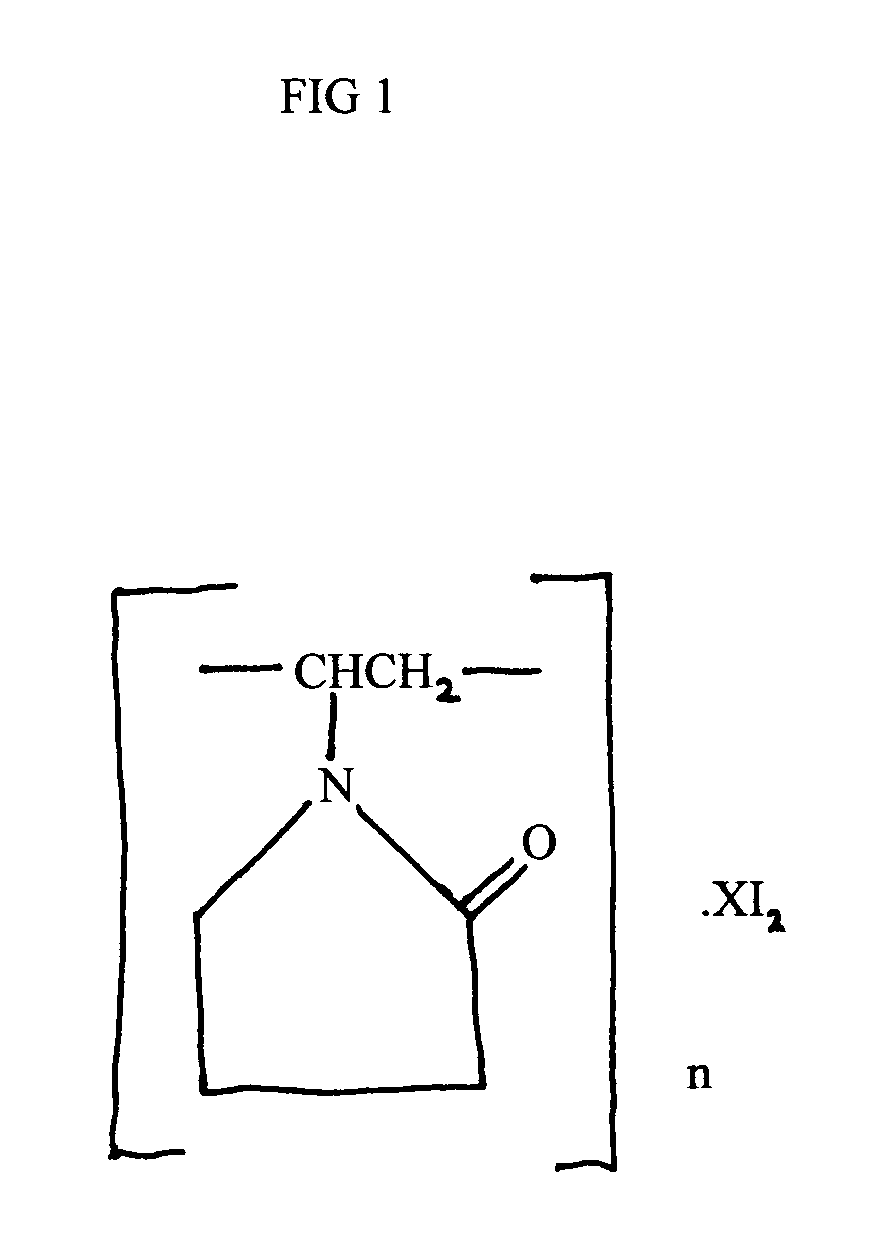
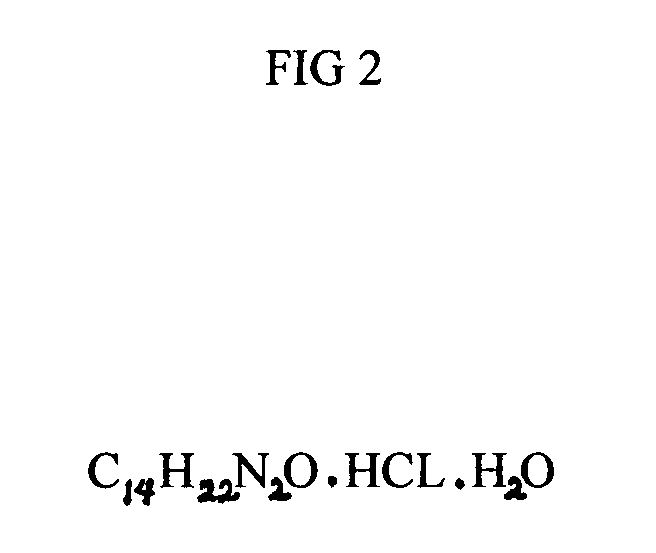
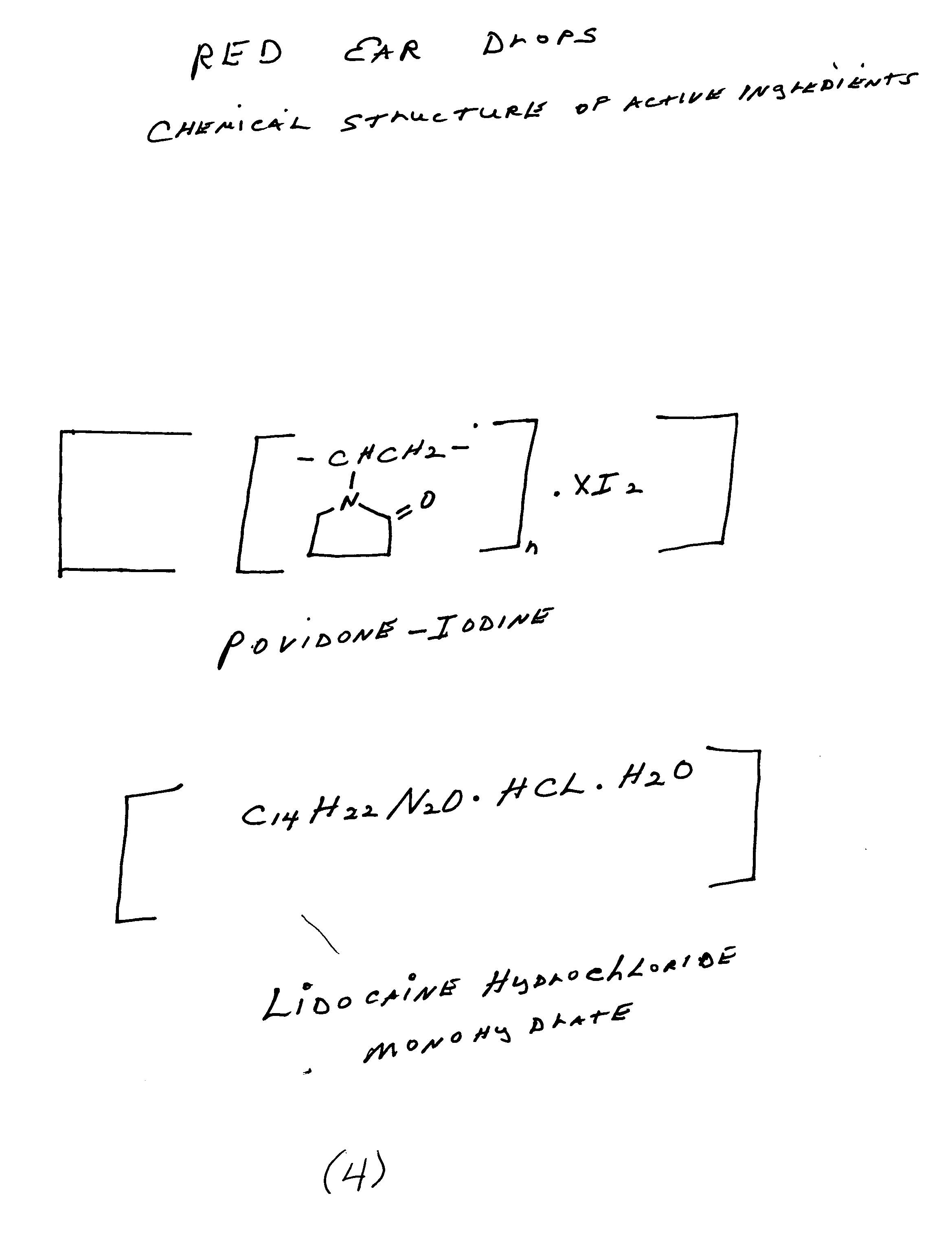
Red ear drops
InactiveUS20060057226A1Effective ear dropRelieve painBiocideOrganic active ingredientsAnesthetic AgentProtozoa
My invention, Red Ear Drops, is a combination of povidone-iodine and lidocaine hydrochloride monohydrate. The prior entity, povidone-iodine, is effective against many pathogens which include viruses, bacteria, fungi, protozoa, and yeast. The latter drug, lidocaine hydrochloride monohydrate is an effective all purpose, local anesthetic agent. The combination drug product can be an effective and safe treatment for many types of ear infections at a reasonable cost to the patient.
Owner:JOHNSON JOHN MOORE
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com