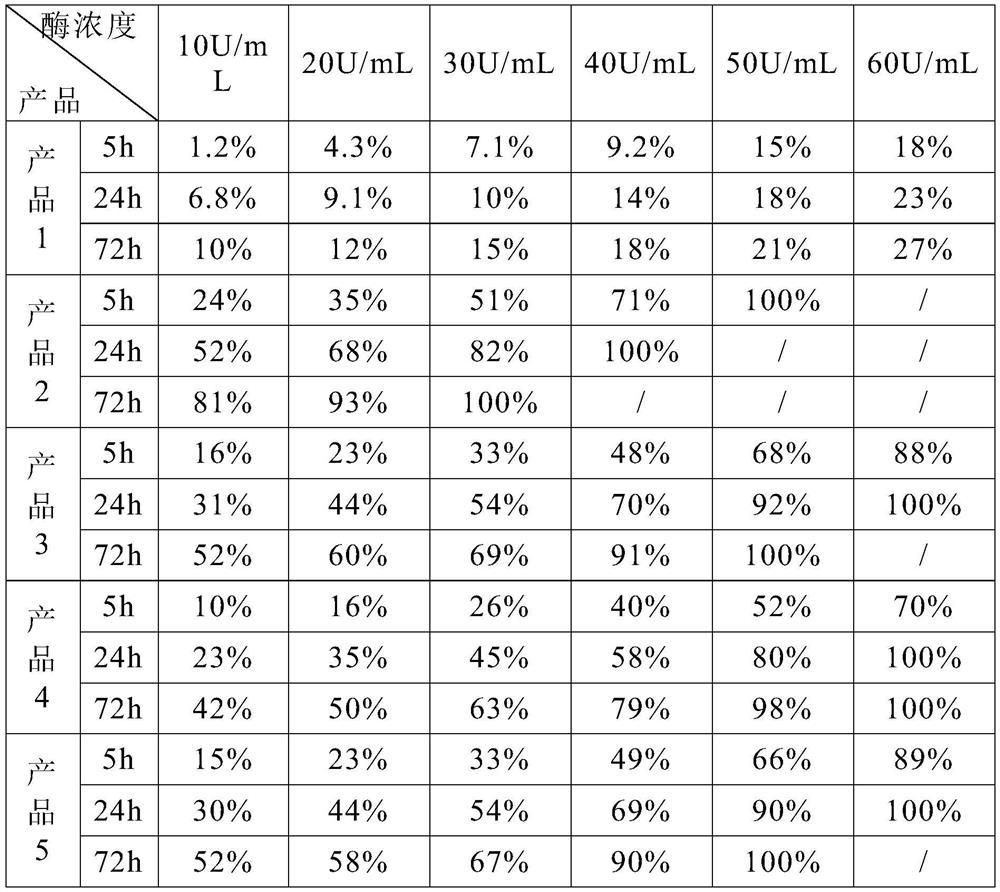
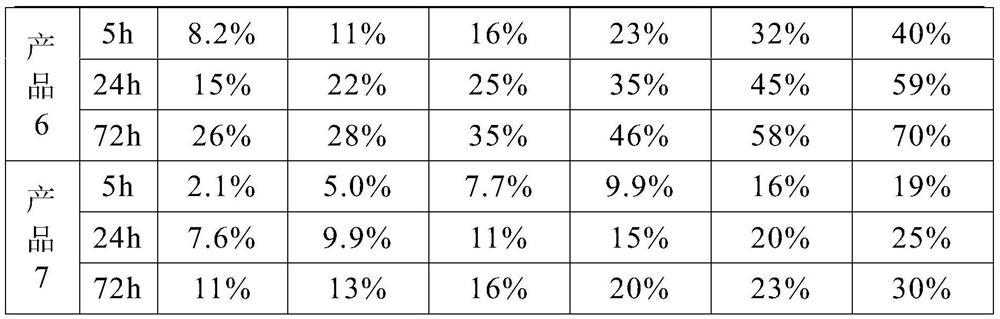
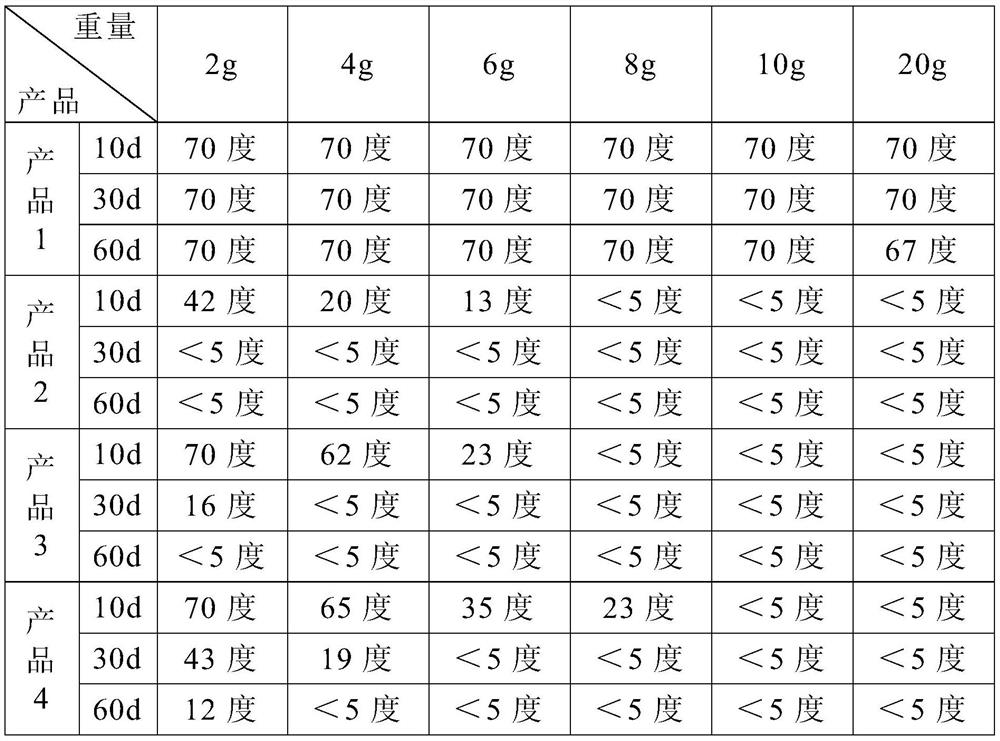
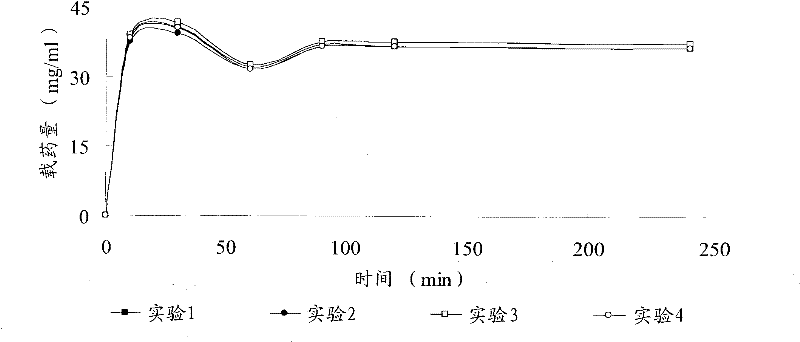
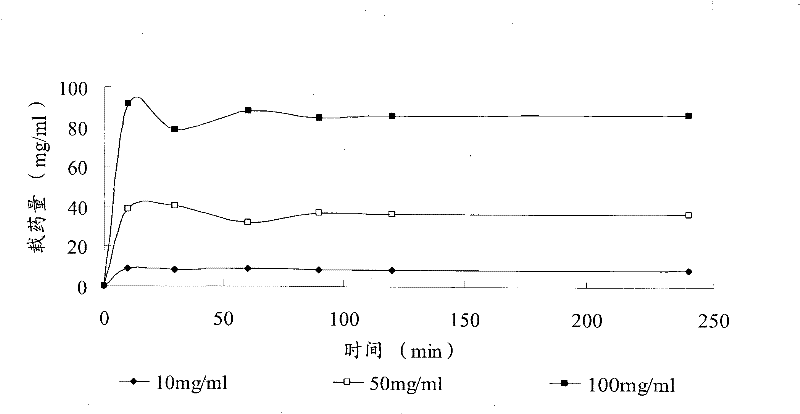
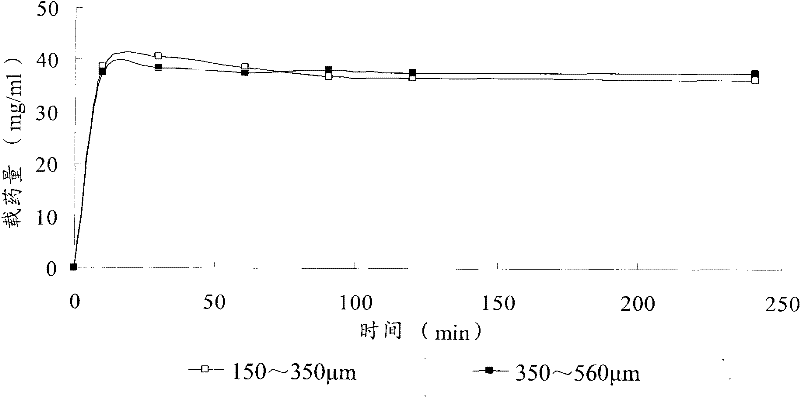
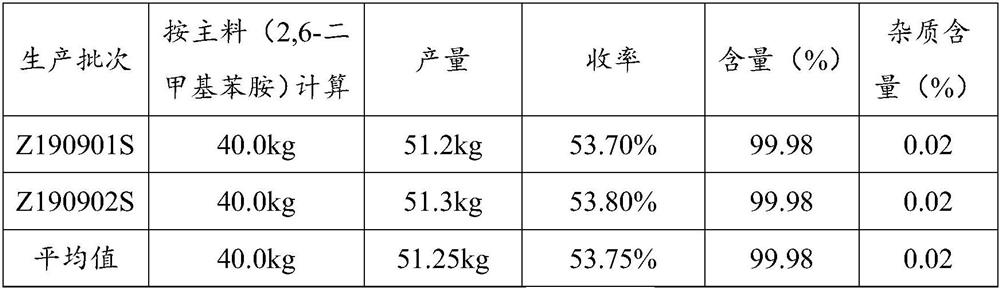
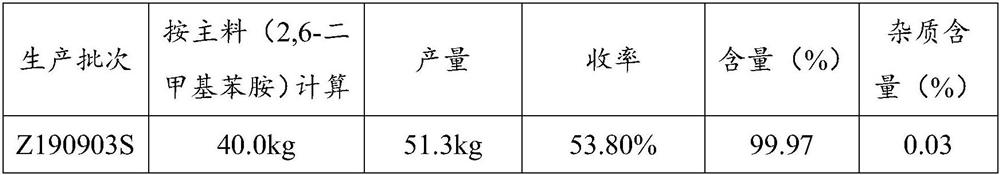
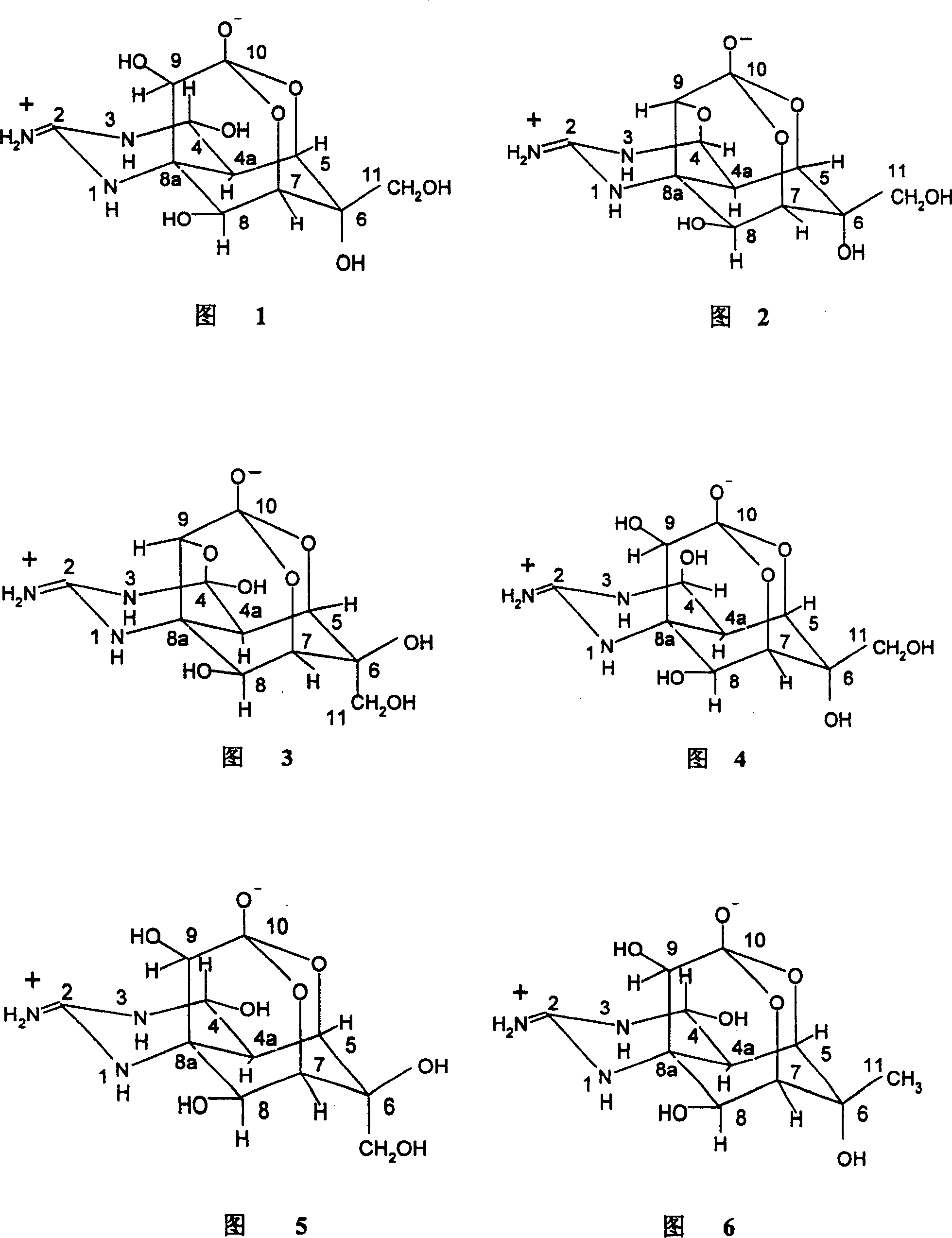
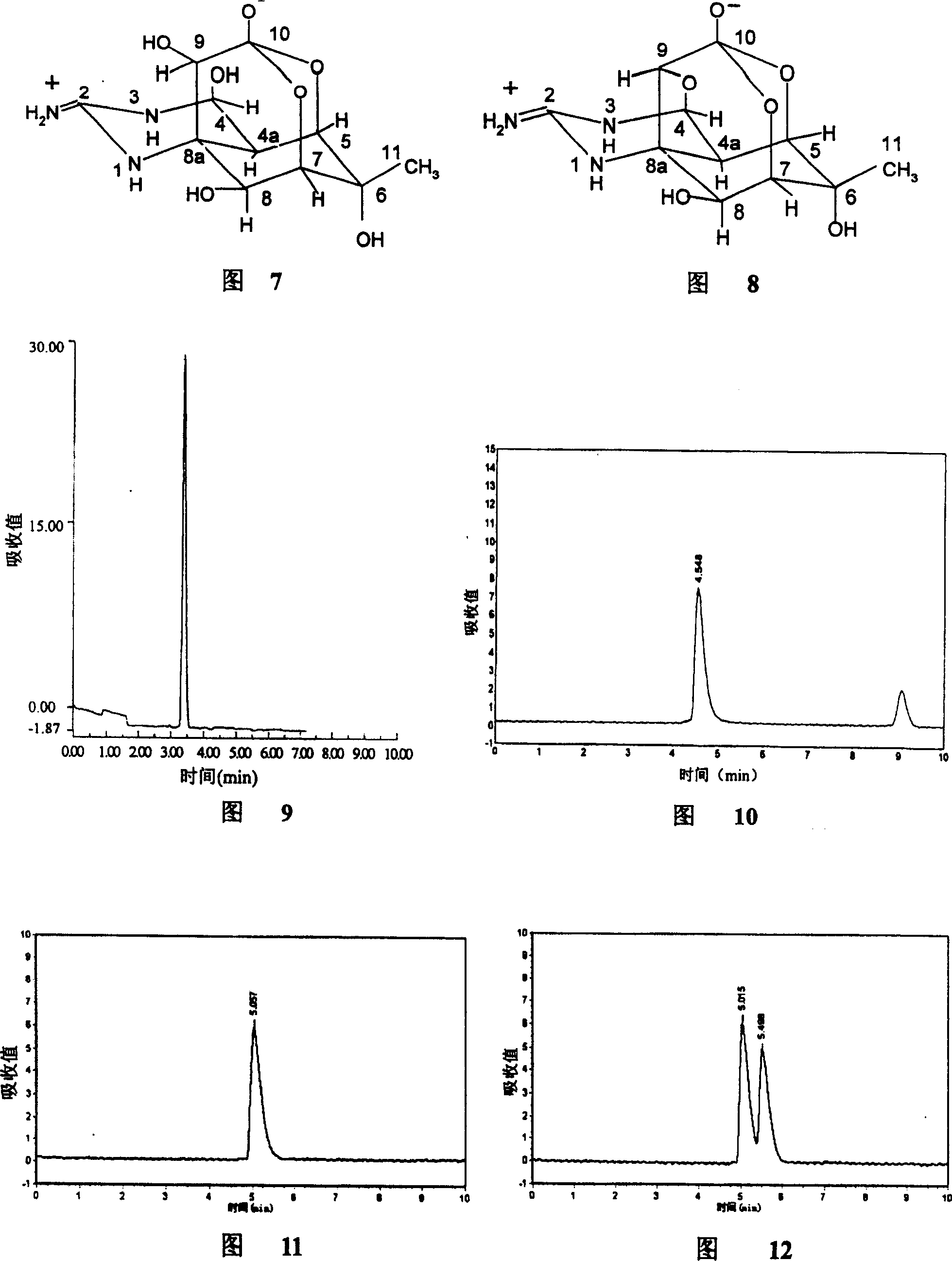
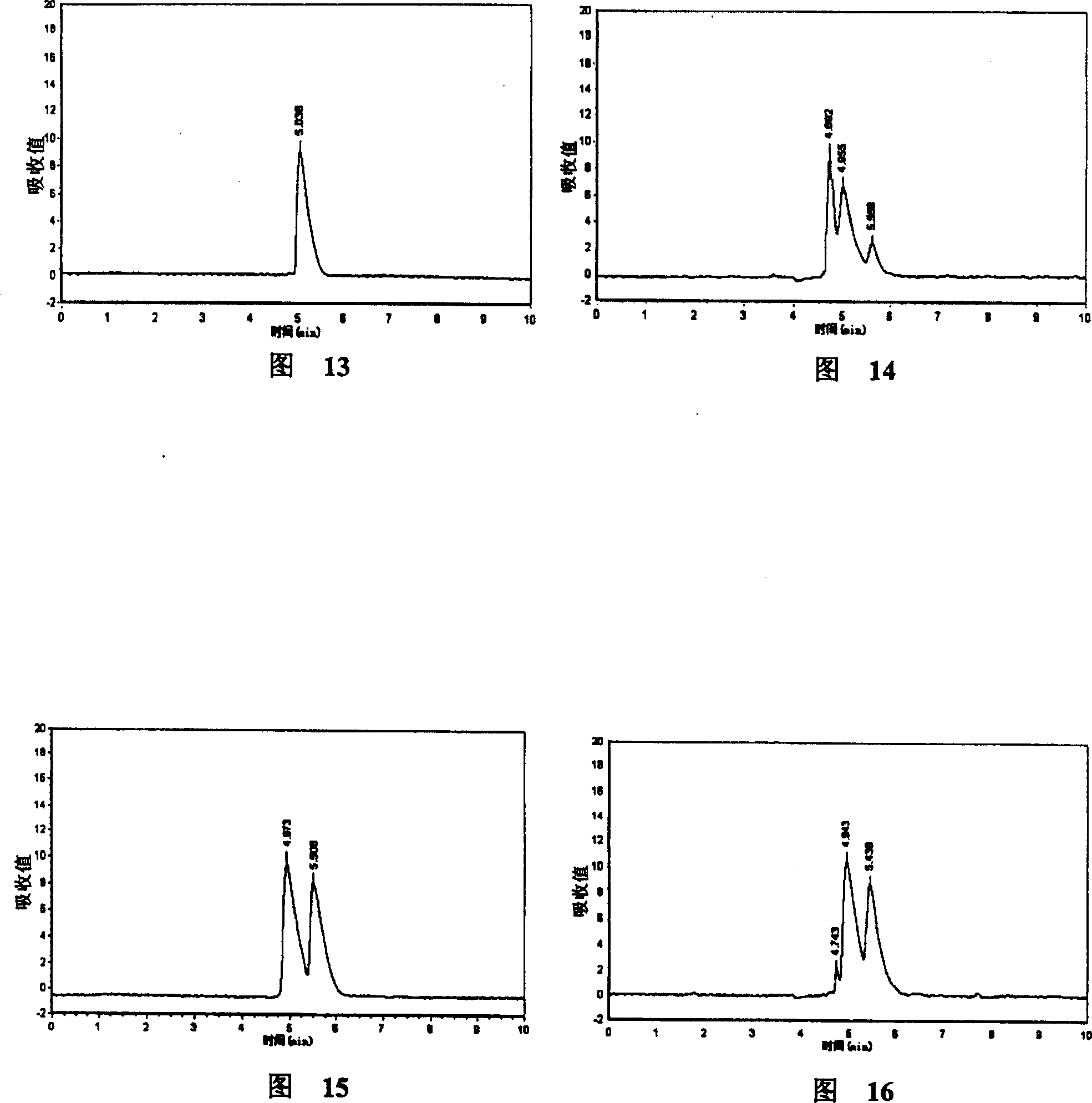
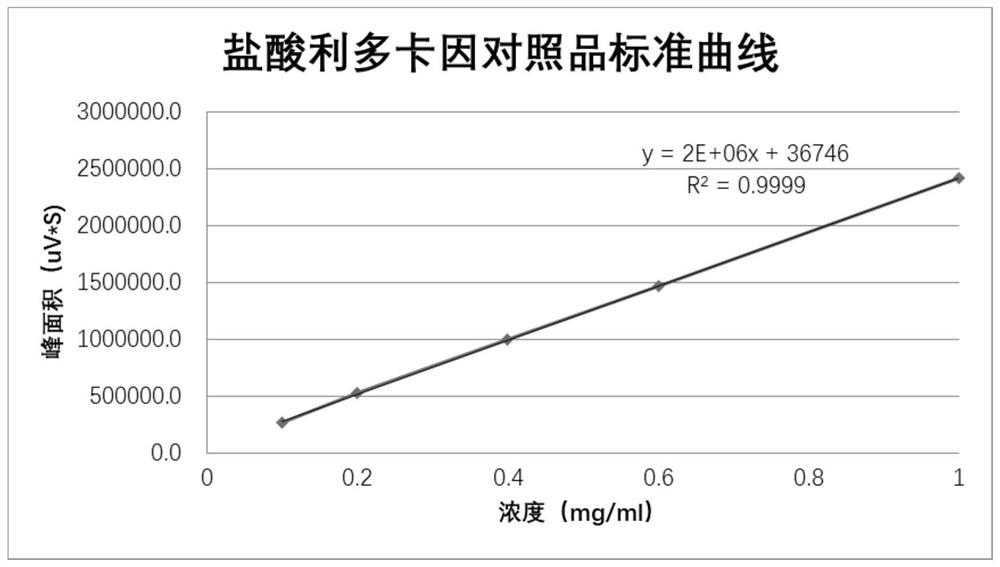
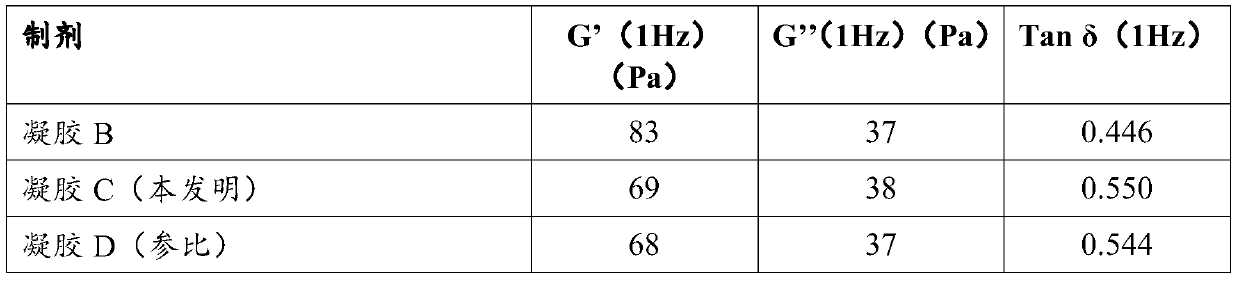
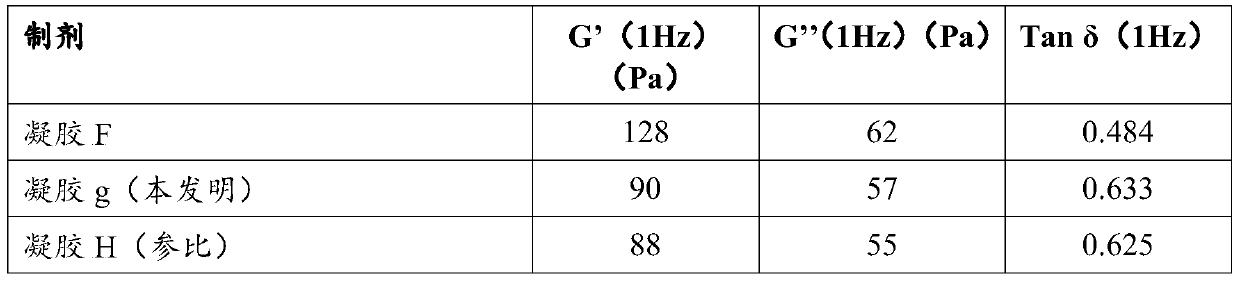
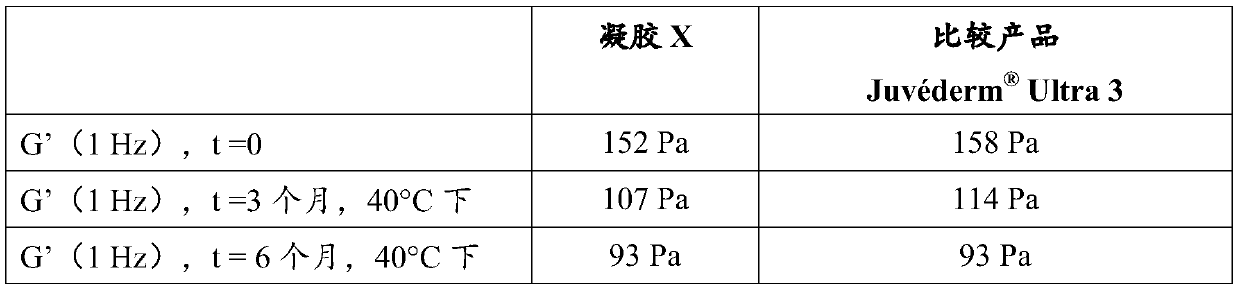
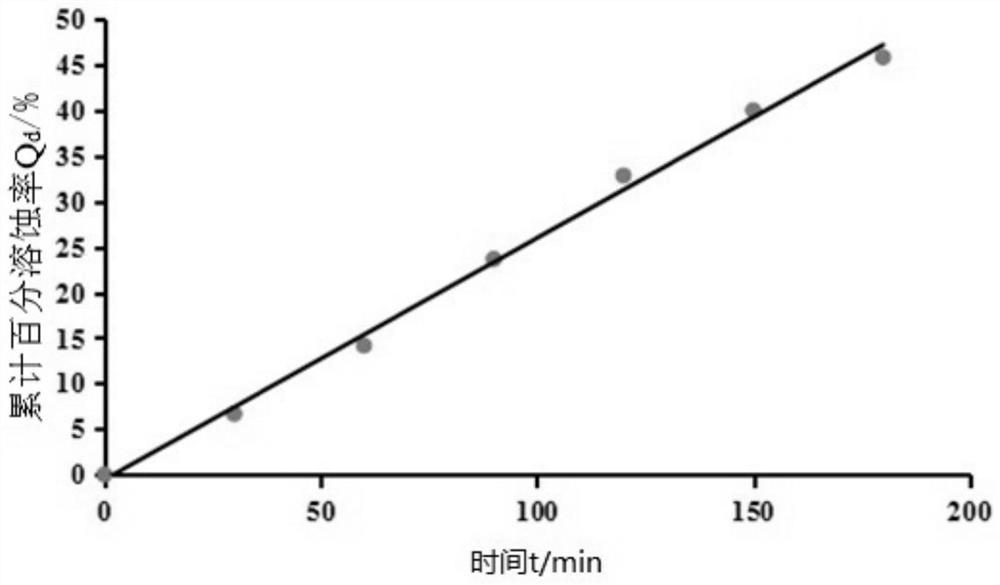
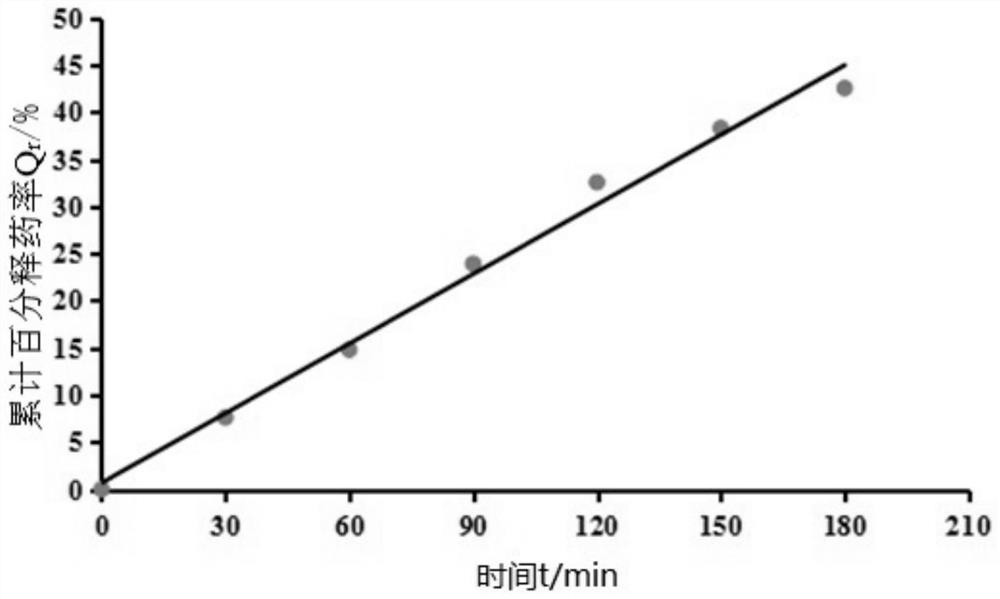
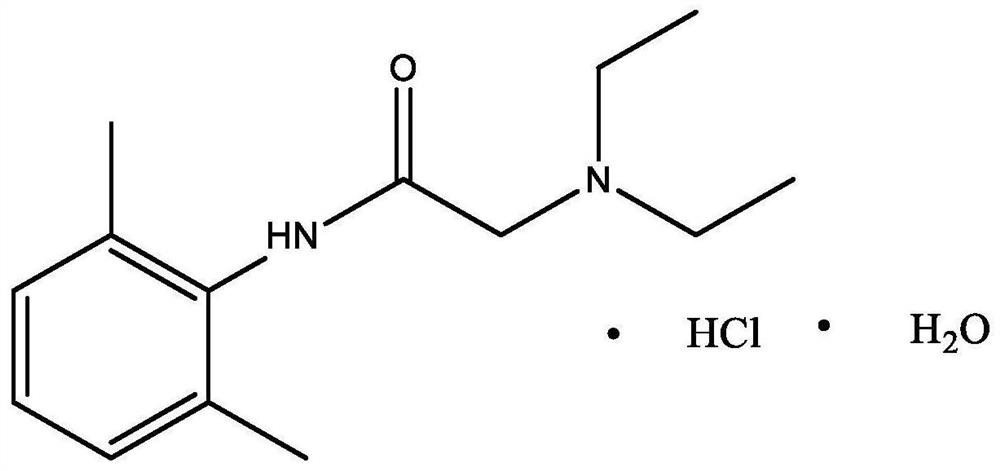
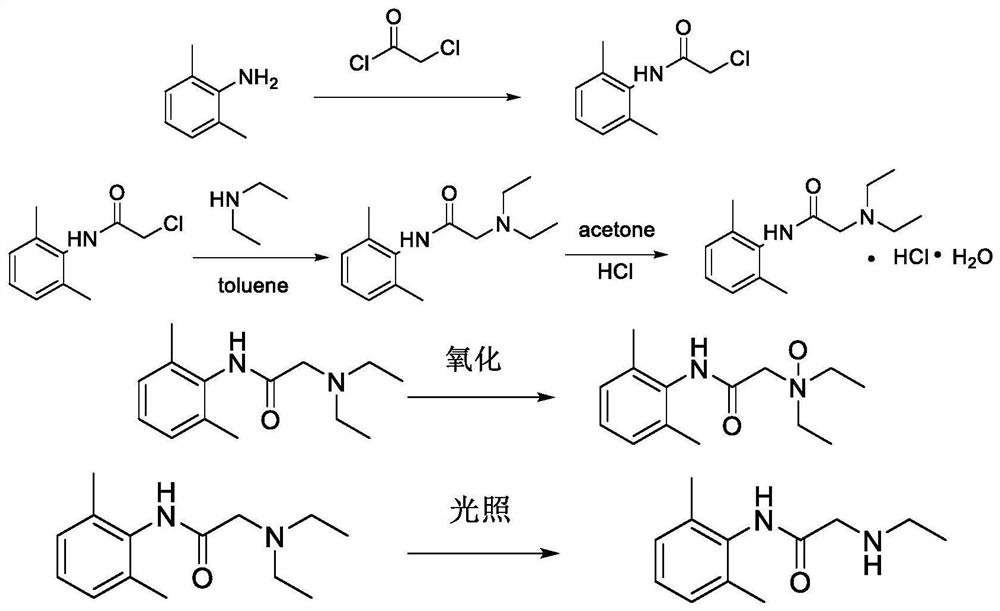
Patents
Literature
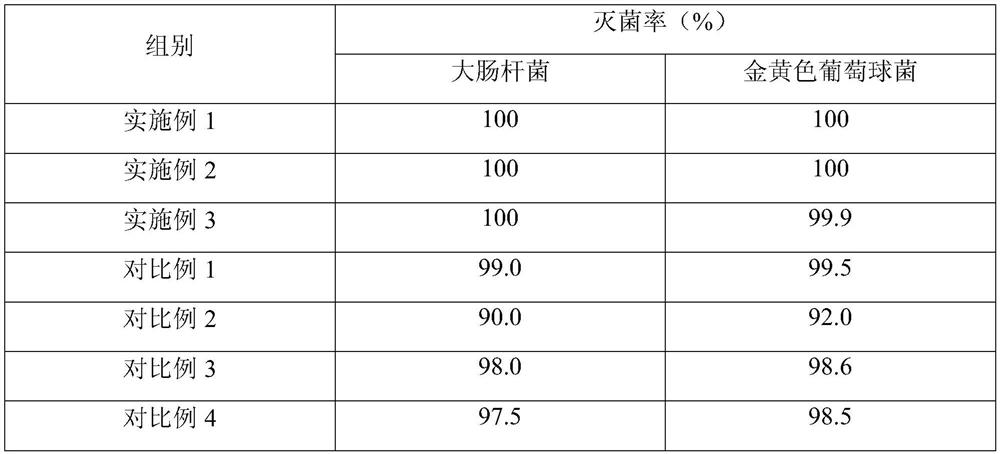
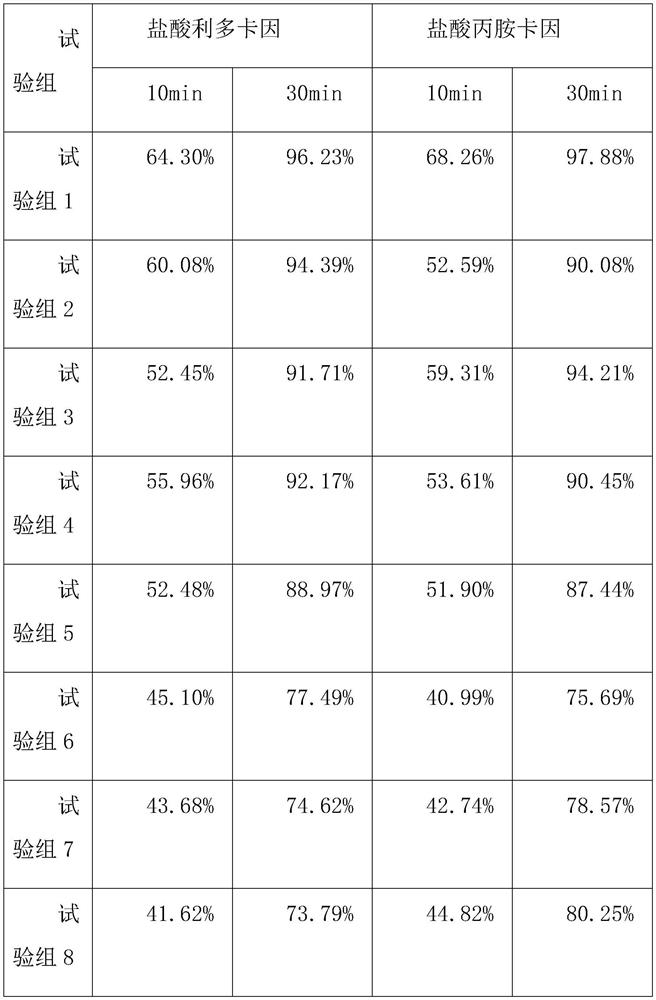
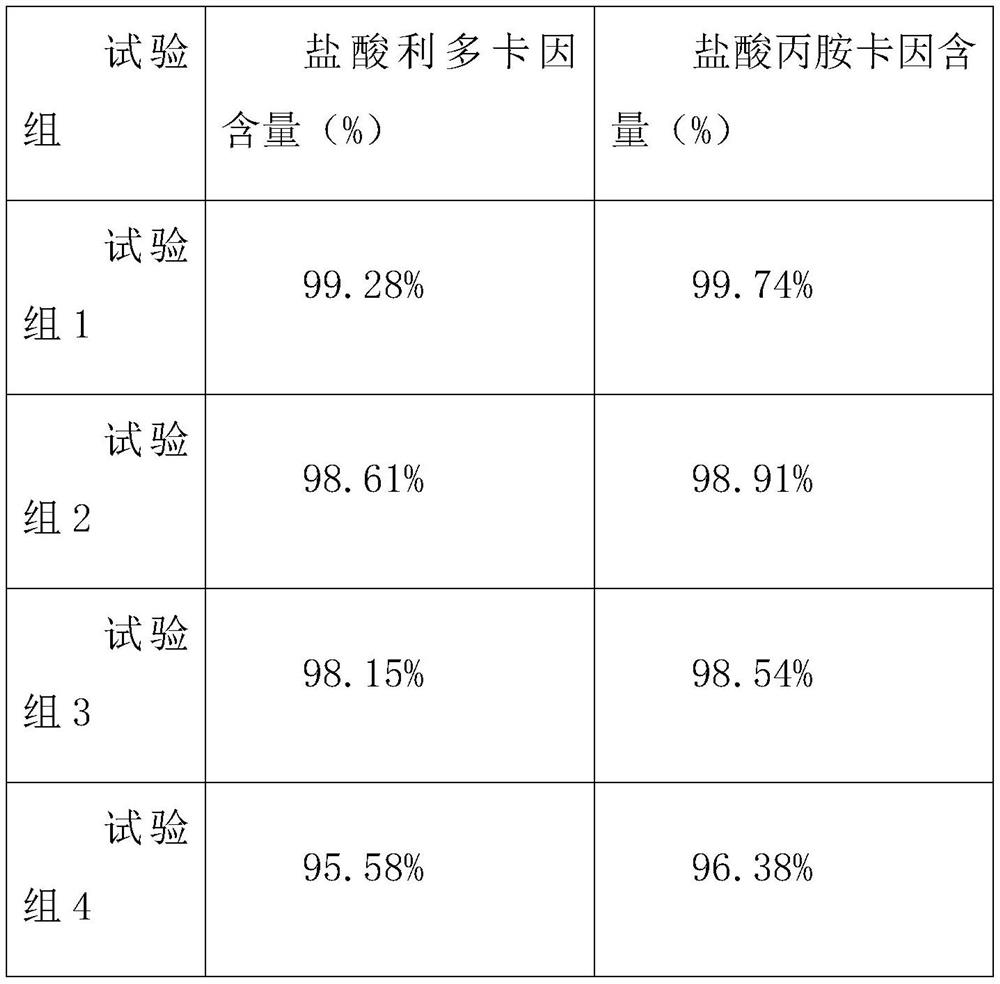
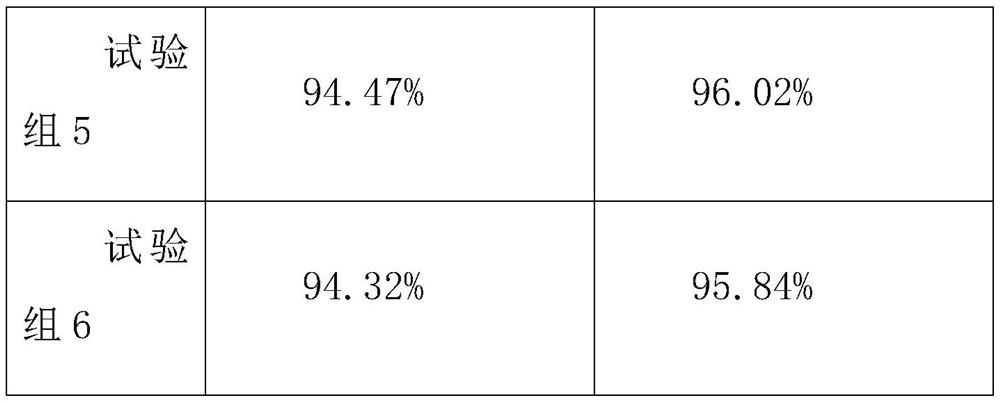
30 results about "Lignocaine hydrochloride" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
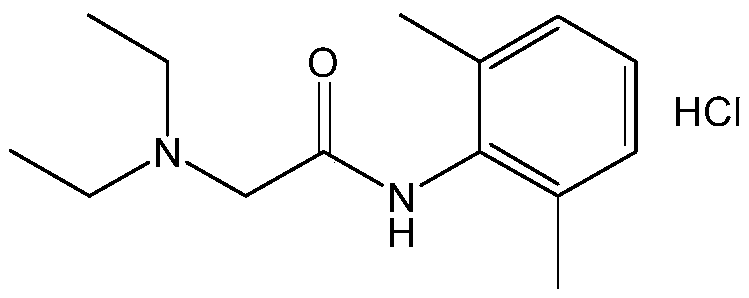
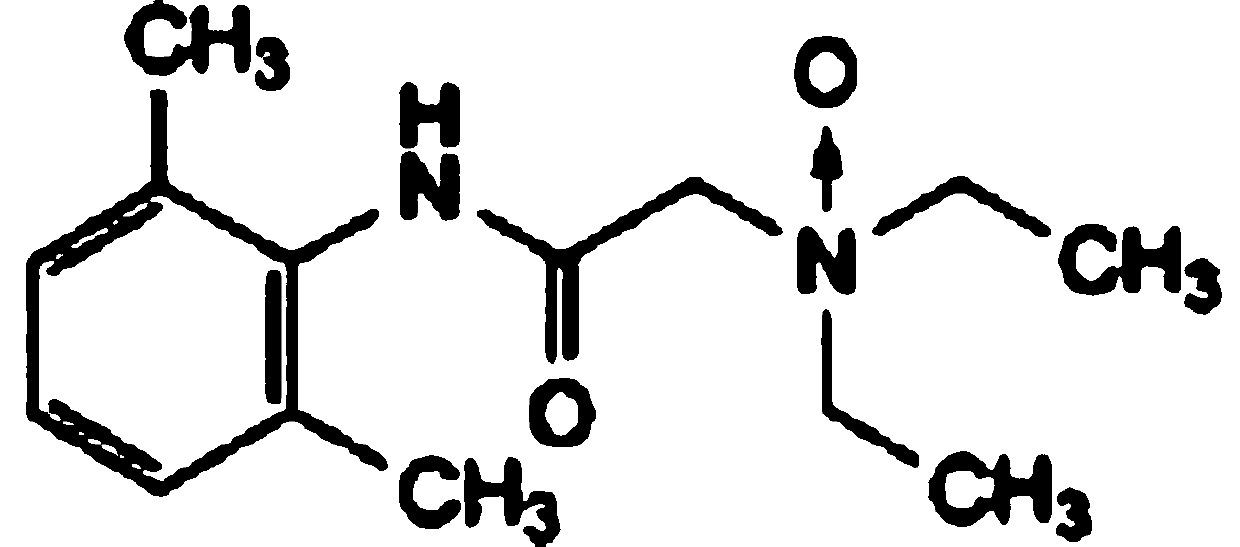
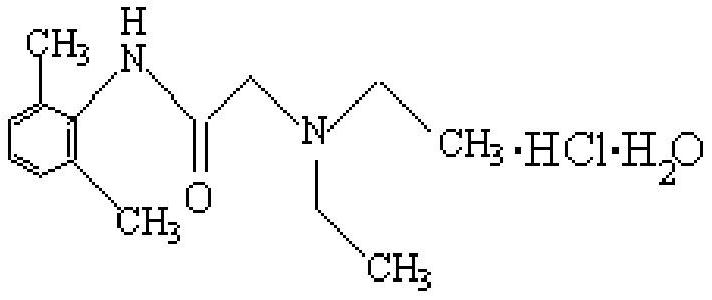
Lidocaine Hydrochloride is the hydrochloride salt from of lidocaine, an aminoethylamide and a prototypical member of the amide class anesthetics. Lidocaine interacts with voltage-gated Na+ channels in the nerve cell membrane and blocks the transient increase in permeability of excitable membranes to Na+.
Method for preparing lidocaine hydrochloride
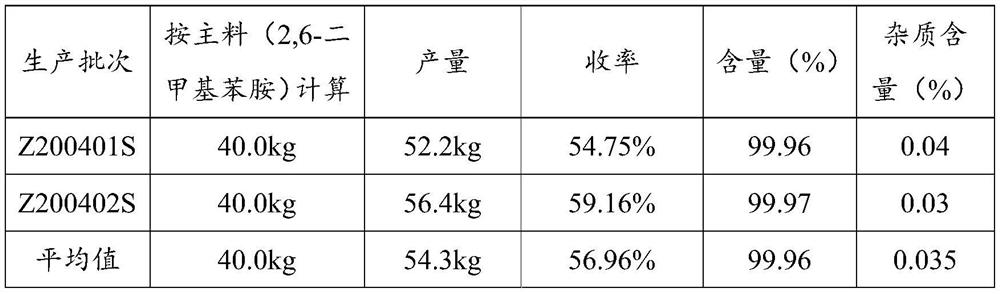
ActiveCN105294477AThe synthesis process is simpleHigh purityOrganic compound preparationCarboxylic acid amides preparationDimethylaniline N-oxideOrganic layer
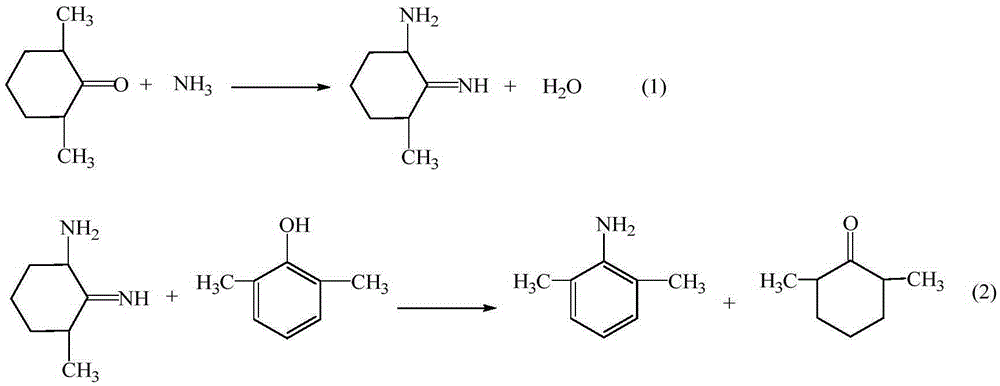
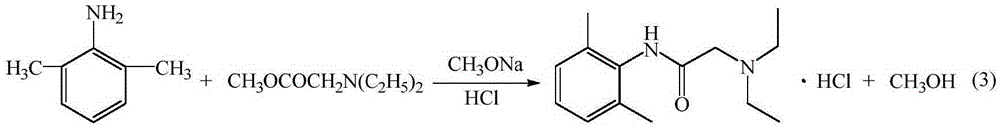
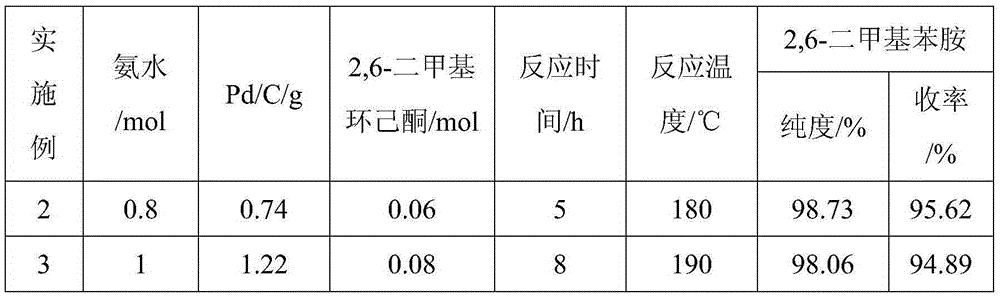
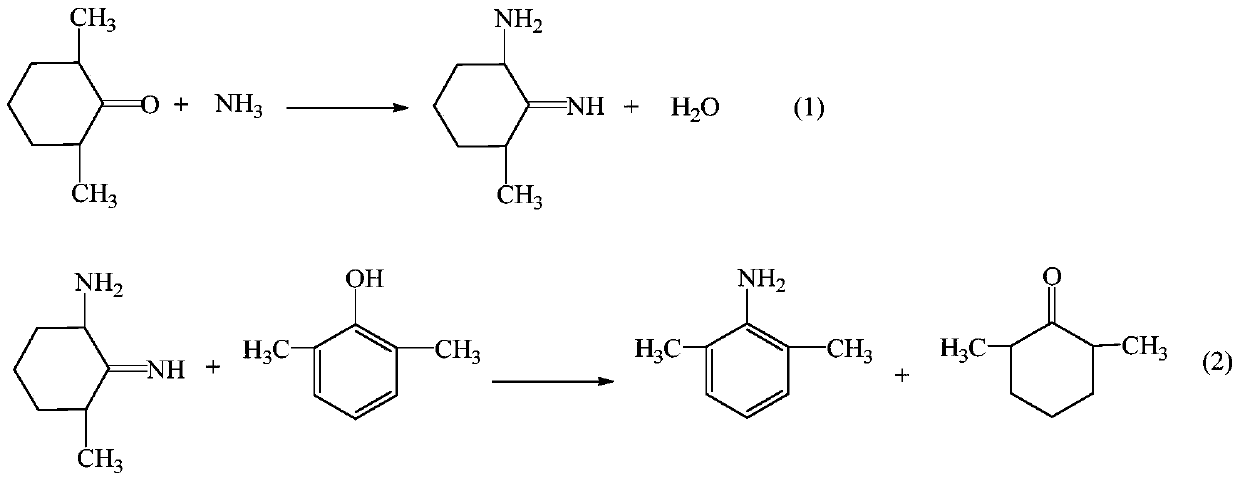
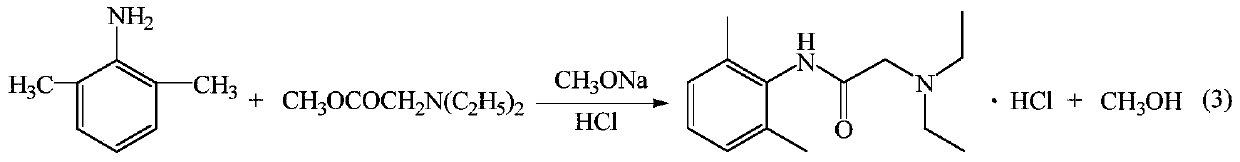
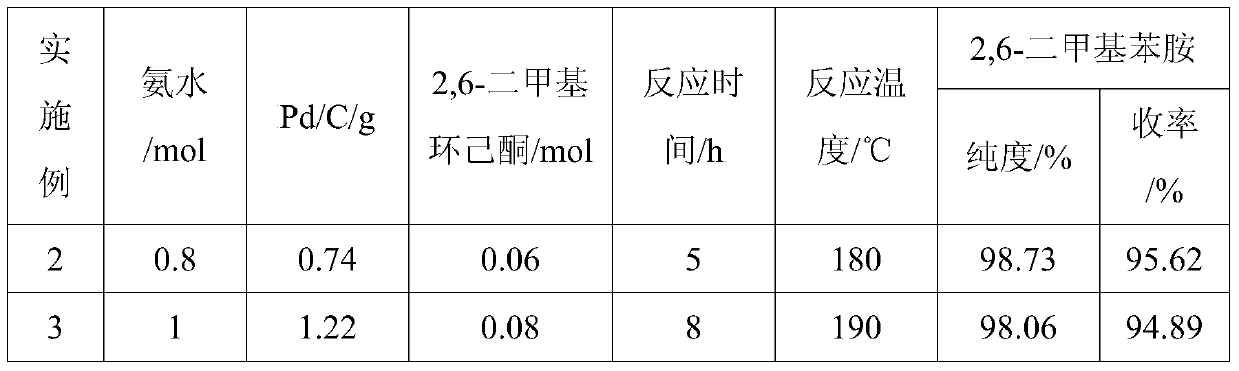
The invention provides a method for preparing lidocaine hydrochloride, and belongs to the technical field of anesthetic synthesis. The method comprises the following steps: by taking 2,6-xylenol as a raw material, Pd / C as a main catalyst and 2,6-dimethylcyclohexanone as a promoter, performing liquid phase amination with ammonia water at high temperature, thereby obtaining a midbody 2,6-dimethylaniline; enabling sodium methylate, 2,6-dimethylaniline and N,N-lignocaine methyl acetate as raw materials to react at 90-95 DEGC, distilling while reaction is performed to remove methanol till no methanol can be evaporated out, continuously reacting for 30 minutes, cooling to the room temperature, adding dichloroethane, washing with water, and leaving to stand to layer, thereby obtaining an organic layer, namely, a lidocaine based dichloroethane solution; further adding hydrochloric acid into the lidocaine based dichloroethane solution, adjusting the pH value to be 3.5-4 by using hydrogen chloride, adding activated carbon to reflux for 20-40 minutes, filtering, concentrating the filtrate, cooling, crystallizing, and dying, thereby obtaining lidocaine hydrochloride. The lidocaine hydrochloride prepared by using the method is simple in synthesis process and high in product purity, that is, the purity can be greater than 99%, and the total yield is greater than 84%.
Owner:ZHEJIANG ESIGMA BIOTECH CO LTD
Preparation method of lidocaine hydrochloride
PendingCN110642738AAvoid multiple purificationsSimple methodOrganic compound preparationCarboxylic acid amides preparationLignocaine hydrochlorideDimethylaniline N-oxide
The invention relates to a preparation method of lidocaine hydrochloride, which comprises the following steps: carrying out acylation reaction by using 2, 6-dimethylaniline and chloroacetyl chloride as raw materials, directly adding diethylamine into the system to carry out amination reaction after the reaction is finished, filtering the product, and adding hydrochloric acid into the filtrate to carry out salification reaction. The preparation method of lidocaine hydrochloride provided by the invention is a one-pot method, avoids repeated purification of an intermediate product in a traditional process, and is simple in process, mild in condition, easy to control, high in product yield and high in purity.
Owner:BENGBU BBCA MEDICINE SCI DEV
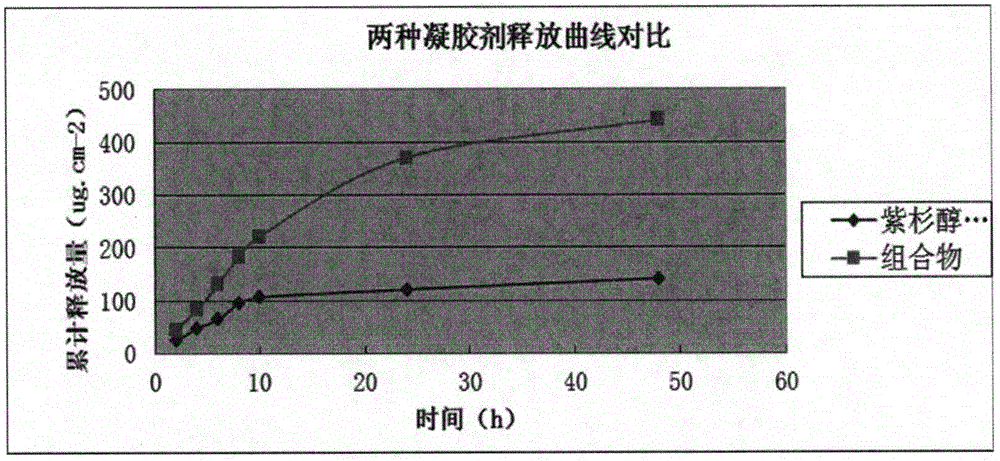
Anti-rheumatoid arthritis drug gel containing paclitaxel liposome and preparation method of gel
InactiveCN104688721AQuick Pain ReliefImprove bioavailabilityOrganic active ingredientsAntipyreticTreatment effectBioavailability
The invention relates to anti-rheumatoid arthritis drug gel containing paclitaxel liposome and a preparation method of the gel. The gel is prepared from main drugs and blank gel, wherein the main drugs comprise paclitaxel liposome and lidocaine hydrochloride; the mass volume ratio of the paclitaxel liposome to lidocaine hydrochloride is 5:100-20:100; and the mass volume ratio of lidocaine hydrochloride to the blank gel is 1:100-5:100. According to the requirements of evidence-based medicine, a drug paclitaxel for treating RA and lidocaine are compatible with each other, the paclitaxel is coated by utilizing a liposome technology, the bioavailability is improved, and the prepared gel suitable for directed use at an affected part is adopted. The gel achieves a treatment effect aiming at the pathogenesis of RA, and pain of a patient suffering from RA can be rapidly relieved. A novel therapy approach is provided for vast patients suffering from RA.
Owner:黄萍
Medicinal composition containing ceftriaxone sodium and lidocaine hydrochloride injection
InactiveCN1943578AProduct quality is stable and controllableReasonable formulaAntibacterial agentsPowder deliveryCeftriaxone SodiumDrug
The invention relates to a medicine composition for injection containing ceftriaxone sodium and Lidocaine Hydrochloride, and characterized by said composition contains 50mg-5000mg ceftriaxone sodium, 1mg-100mg Lidocaine Hydrochloride. The composition in said invention is prepared by split charging of germ-free ceftriaxone sodium and Lidocaine Hydrochloride in accordance with specifications.
Owner:GUANGZHOU PUIS PHARMA FACTORY
Preparation method of synergistic tylosin tartrate soluble powder compound medicine
InactiveCN103405463AWide range of indicationsGood curative effectAntibacterial agentsOrganic active ingredientsTrimethoprimSecondary Infections
The invention aims at providing a preparation method of a synergistic tylosin tartrate soluble powder compound medicine. The synergistic tylosin tartrate soluble powder compound medicine has a rapid and definite curative effect on respiratory system infections and complications of livestock and poultry. The preparation method comprises the following steps of: mixing 0.2 part of trimethoprim and 0.2 part of cosolvent, then crushing, and sieving by using a 120 meshes sieve or a finer sieve; and then uniformly mixing with 1 part of tylosin tartrate, 1 part of kanamycin sulfate and 0.05-0.1 part of pain alleviant by an equivalently successive increase method, thus obtaining the synergistic tylosin tartrate soluble powder compound medicine. The cosolvent is citric acid or succinic acid or the mixture of citric acid and succinic acid and the pain alleviant is procaine hydrochloride or lidocaine hydrochloride or the mixture of procaine hydrochloride and lidocaine hydrochloride. The animal synergistic tylosin tartrate soluble powder compound medicine for injection contains three medical components which are tylosin tartrate, kanamycin sulfate and trimethoprim, can be used for solving the problems that the secondary infection cannot be controlled very well during the treatment of mycoplasma infection due to the narrow antibacterial spectrum of the tylosin tartrate, and the trimethoprim is insoluble in water, has a synergistic effect on tylosin tartrate and kanamycin sulfate and has an obvious medicine curative effect.
Owner:四川联美生物药业有限公司
Composition containing glucosamine as well as preparation method and detection method thereof
The invention discloses an injection containing glucosamine sulfate double salt, the active ingredients of which are glucosamine sulfate double salt and lidocaine hydrochloride. The injection described in the invention is preferably a small-volume injection, and the injection further includes antioxidants, pH regulator and alkaline solvent, the injection of the present invention overcomes the long freeze-drying time of the freeze-dried preparation of glucosamine in the prior art, high energy consumption, and is unfavorable for production, and the freeze-dried preparation of the same batch is different in form and recombination. The defect that there is a large difference in solubility has realized the control of the stability of the injection and related substances, and reduced the side effects caused by the degradation products, thereby meeting the requirements of the safety and stability of the injection. Therefore, the amino acid provided by the present invention The glucose injection can satisfy industrial production, and can be safely and reliably applied in the field of medicine.
Owner:任金山 +1
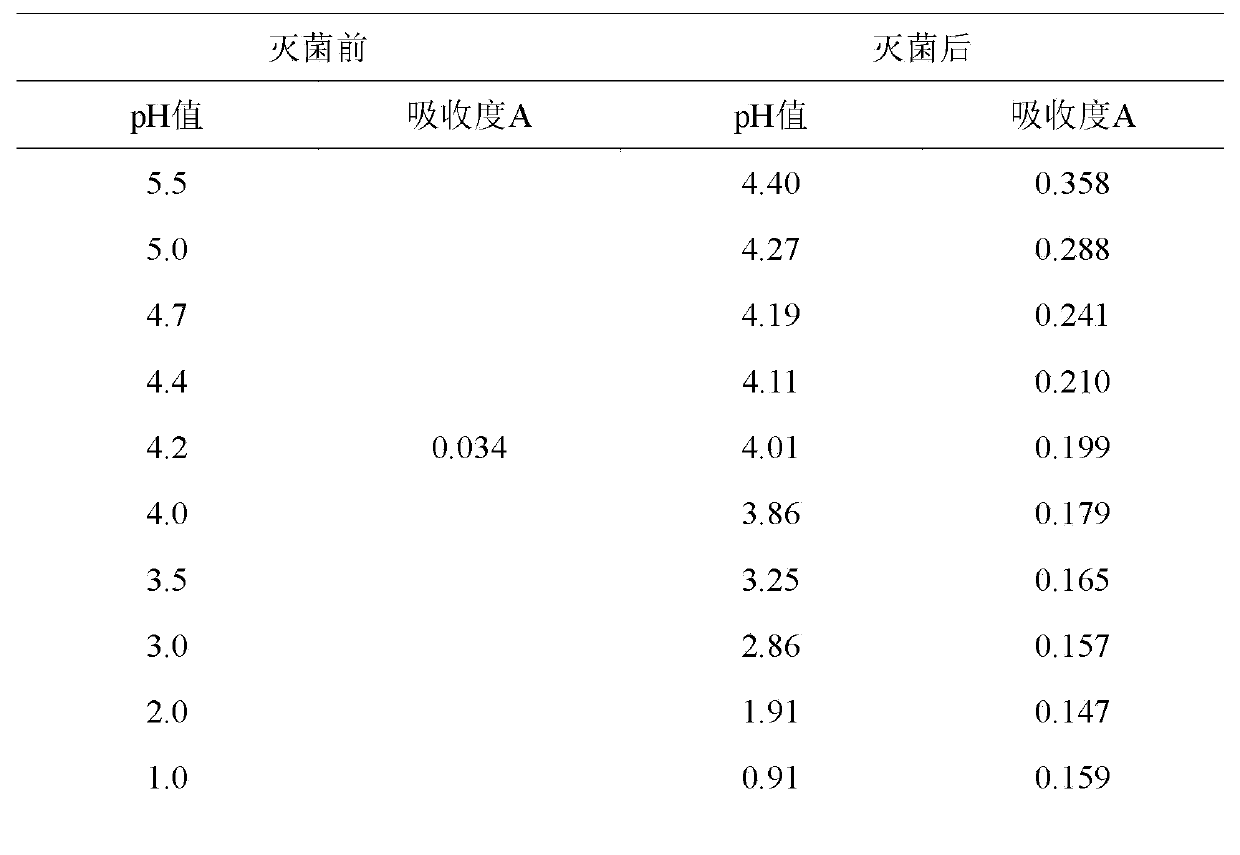
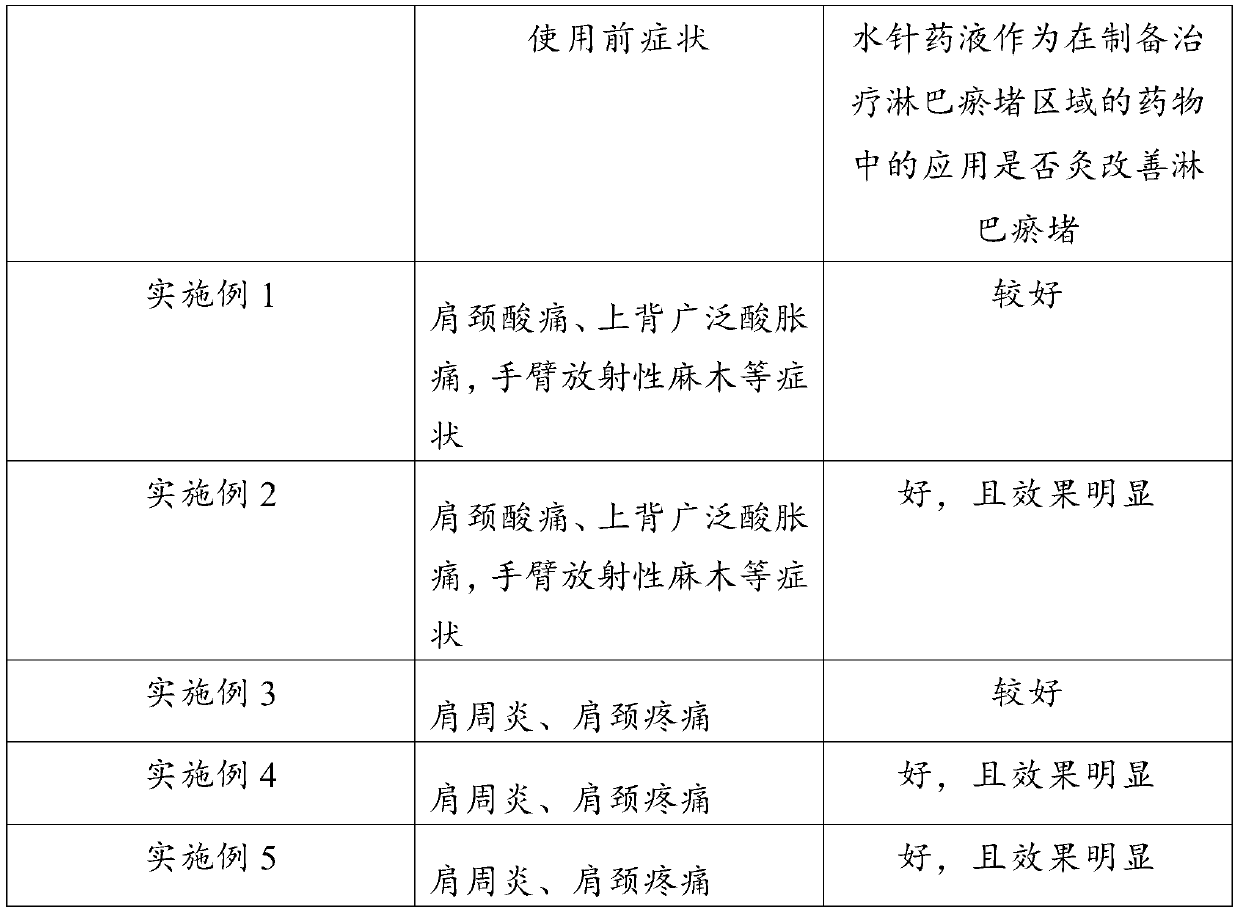
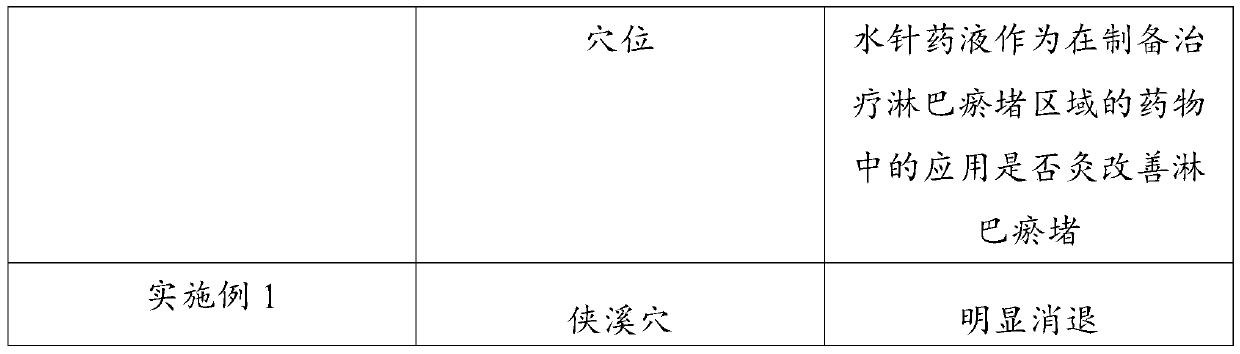
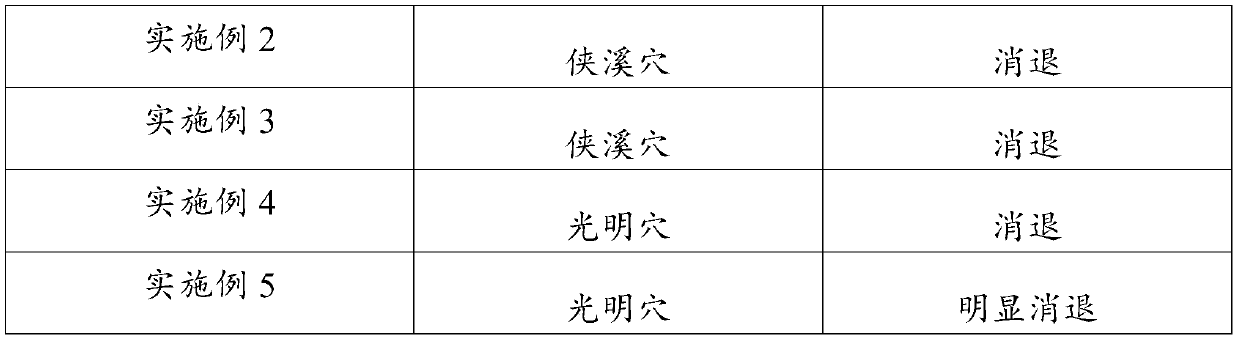
Application of hydro-acupuncture liquid medicine in preparation of medicine for treating lymphatic obstruction area
PendingCN110604834AImprove edemaGood for healthInfusion syringesPharmaceutical delivery mechanismRadix Astragali seu HedysariBovine collagen
The invention discloses an application of a hydro-acupuncture liquid medicine in preparation of a medicine for treating lymphatic obstruction area. The hydro-acupuncture liquid medicine comprise liquid medicine, wherein the liquid medicine comprises the following raw material components in parts by weight: 1 to 3 parts of a medical collagen filling agent, 2 to 6 parts of a Radix Astragali injection, 2 to 6 parts of a compound Chinese angelica injection and 100 to 300 parts of normal saline, wherein the medical collagen filling agent is a normal saline suspension of 3.5% of bovine collagen and0.3% of lidocaine hydrochloride. The liquid medicine is injected into acupuncture points in a lymphatic obstruction area, so that qi and blood circulation is increased, channels and collaterals and lymph are dredged, and stasis toxin is eliminated.
Owner:上海欧邦医疗管理有限公司
Preparation process of anesthetic drug composition
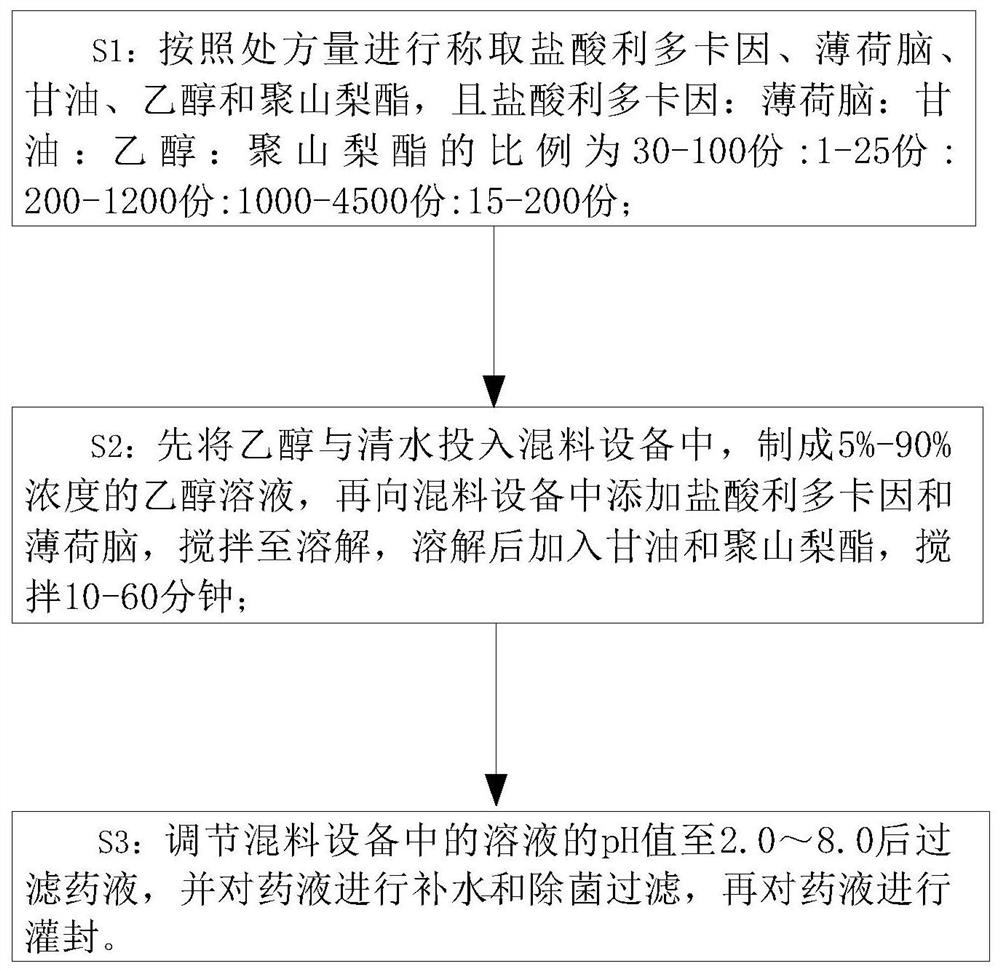
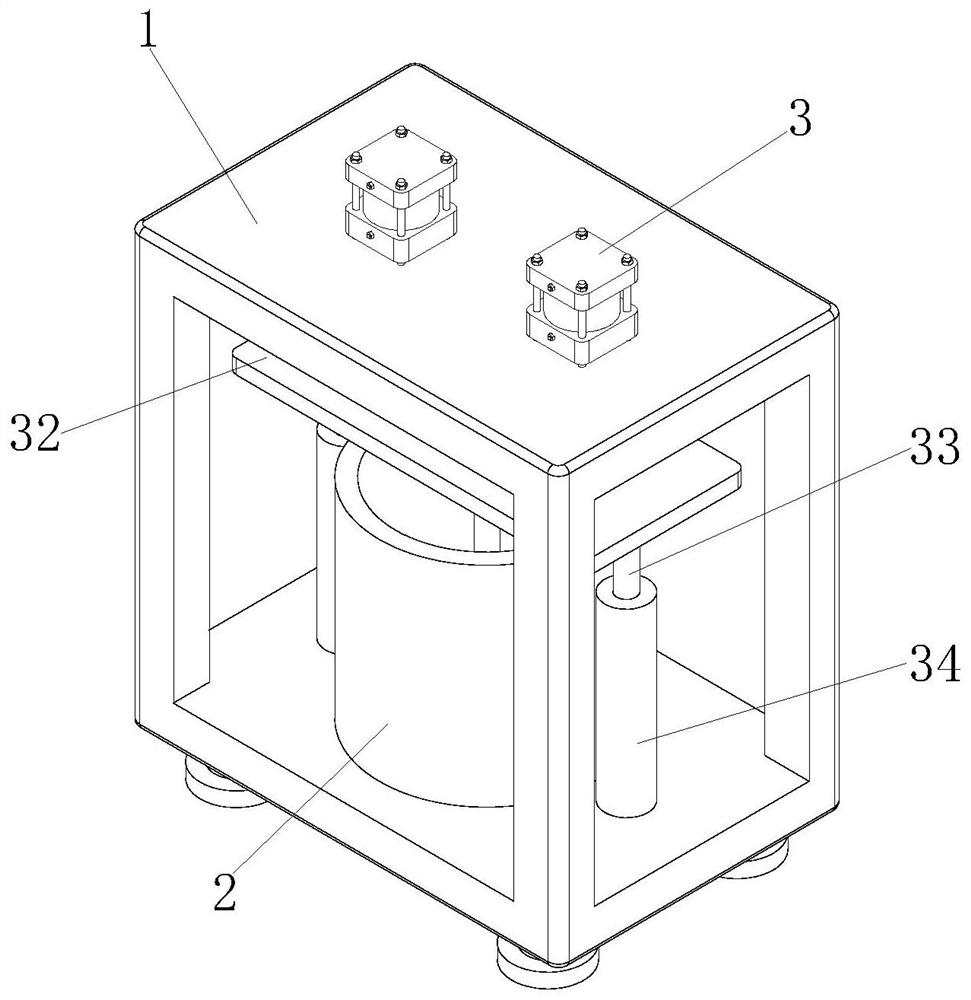
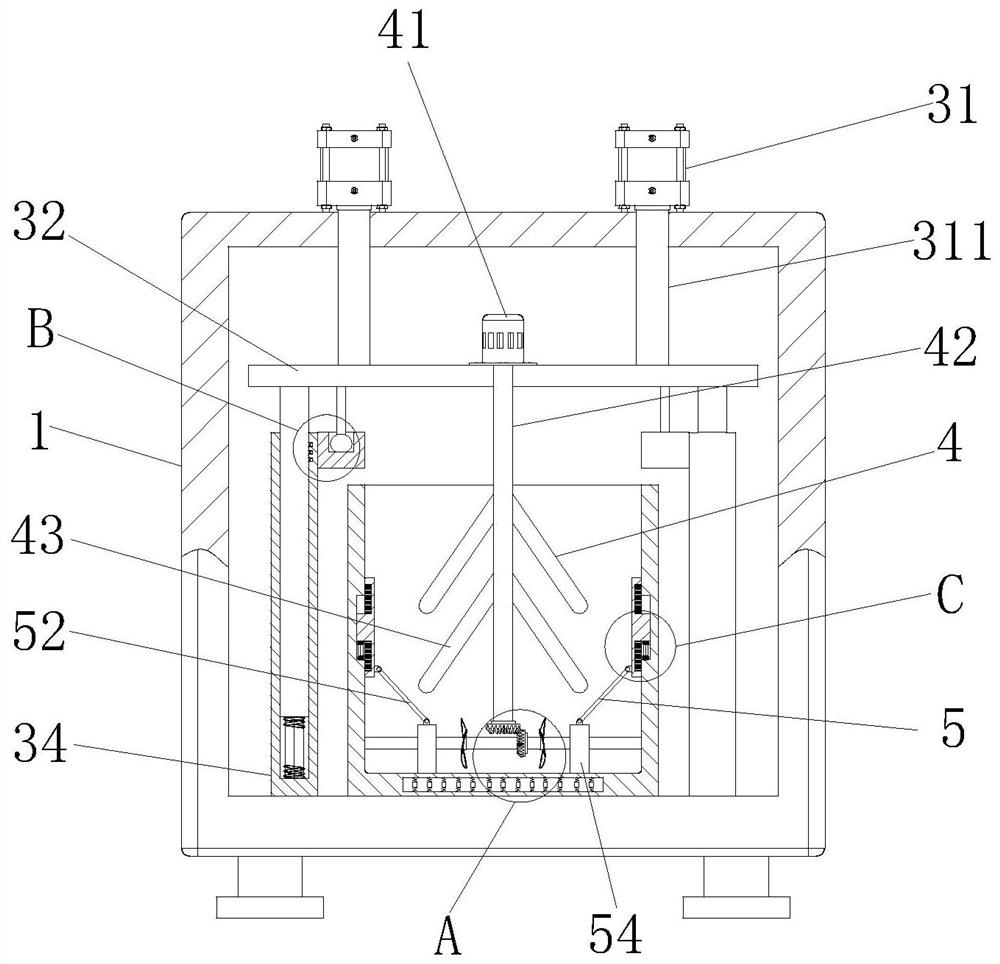
PendingCN112494470AGood anesthesiaAvoid harmOrganic active ingredientsRotary stirring mixersGlycerolDrug efficiency
The invention relates to the technical field of anesthetic drugs, and particularly relates to a preparation process of an anesthetic drug composition. The preparation process comprises the following steps: S1, weighing lidocaine hydrochloride, menthol, glycerol, ethanol and polysorbate according to the prescription amount; wherein the ratio of the lidocaine hydrochloride to the menthol to the glycerol to the ethanol to the polysorbate is (30-100 parts): (1-25 parts): (200-1, 200 parts): (1, 000-4, 500 parts): (15-200 parts); S2, putting the ethanol and clear water into mixing equipment to prepare an ethanol solution with the concentration of 5%-90%, adding the lidocaine hydrochloride and the menthol into the mixing equipment, stirring the materials until dissolved, adding the glycerol andthe polysorbate after dissolving, and carrying out stirring for 10-60 minutes; and S3, adjusting the pH value of the solution in the mixing equipment to 2.0-8.0, then filtering the liquid medicine, carrying out water adding and aseptic filtration on the liquid medicine, and then carrying out encapsulation on the liquid medicine. The prepared anesthetic drug is better in drug effect, the problem that the traditional anesthetic drugs are poor in anesthetic effect is solved, and a patient is prevented from being injured due to insufficient anesthesia.
Owner:哈尔滨医大药业股份有限公司
A kind of preparation method of lidocaine hydrochloride
ActiveCN105294477BThe synthesis process is simpleHigh purityOrganic compound preparationCarboxylic acid amides preparationSodium methoxideCyclohexanone
The invention provides a preparation method of lidocaine hydrochloride, which belongs to the technical field of anesthetic synthesis. The preparation method uses 2.6-xylenol as a raw material, adopts Pd / C as the main catalyst, 2,6-dimethylcyclohexanone as a co-catalyst, and performs liquid-phase amination with ammonia water at high temperature to obtain the intermediate 2,6 -Dimethylaniline; then use sodium methoxide, 2,6-dimethylaniline and N,N-diethylaminoacetate methyl ester as raw materials, react at 90-95°C, distill and remove methanol while reacting until it is free After the methanol is distilled off, continue to react for 30 minutes, cool to room temperature, add dichloroethane, wash with water, leave to stand and separate, the organic layer is the dichloroethane solution of lidocaine base; Add hydrochloric acid to the ethyl chloride solution, then adjust the pH to 3.5-4 with hydrogen chloride, add activated carbon to reflux for 20-40 minutes, filter, and the filtrate is concentrated, crystallized by cooling, and dried to obtain lidocaine hydrochloride. The lidocaine hydrochloride of the present invention has a simple synthesis process, a product purity of more than 99%, and a total yield of more than 84%.
Owner:ZHEJIANG ESIGMA BIOTECH CO LTD
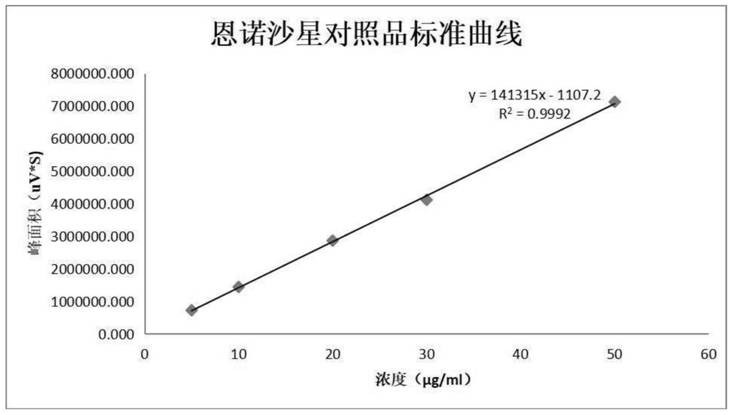
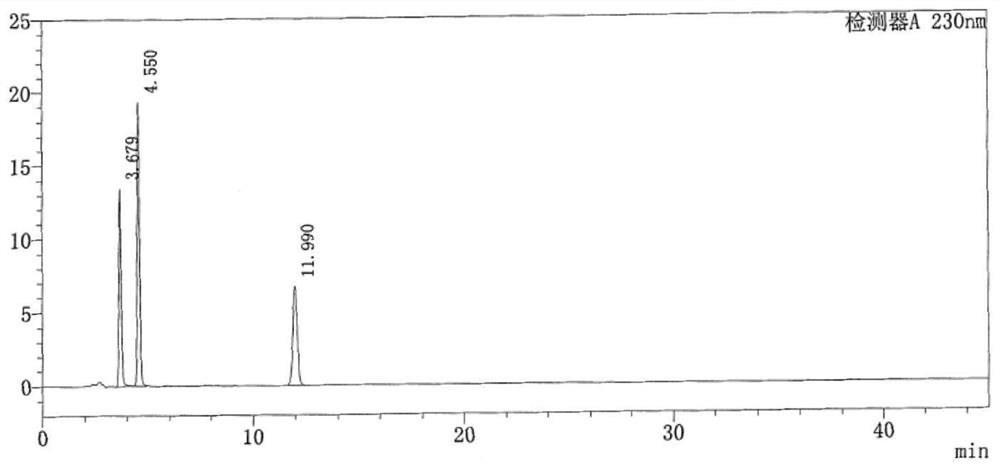
Method for determining lidocaine hydrochloride related substances by high performance liquid chromatography
The invention discloses a method for determining lidocaine hydrochloride related substances by high performance liquid chromatography, and relates to the field of drug detection, the chromatographic conditions are as follows: octadecylsilane chemically bonded silica bonded with polar groups is selected as a filler as a stationary phase; wherein the particle size of the chromatographic column is 5[mu]m, the detection wavelength ranges from 225 nm to 235 nm, the flow rate is 0.8 to 1.2 ml / min, the column temperature is 30-40 DEG C, and the mobile phase is prepared from the following componentsin parts by mass: 50-80 parts of 0.035 mol / L phosphate buffer solution and 20-50 parts of organic solvent. The adopted detector is a high performance liquid chromatograph, more impurities can be detected by the method, and methodological verification proves that the detection result is accurate and reliable.
Owner:TIANJIN PHARMA GROUP XINZHENG
Diclofenac sodium and lidocaine hydrochloride injection and preparation method thereof
InactiveCN111514104AReduce manufacturing costOrganic active ingredientsNervous disorderSulfite saltDisodium Edetate
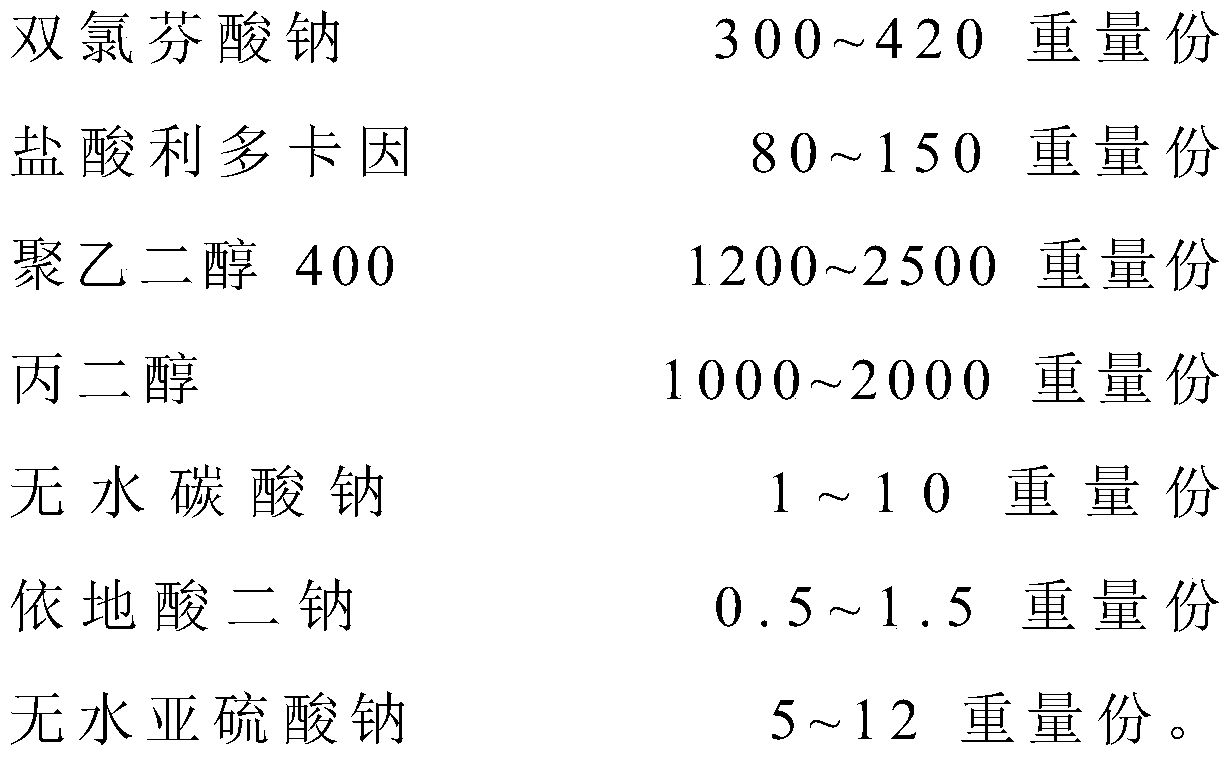
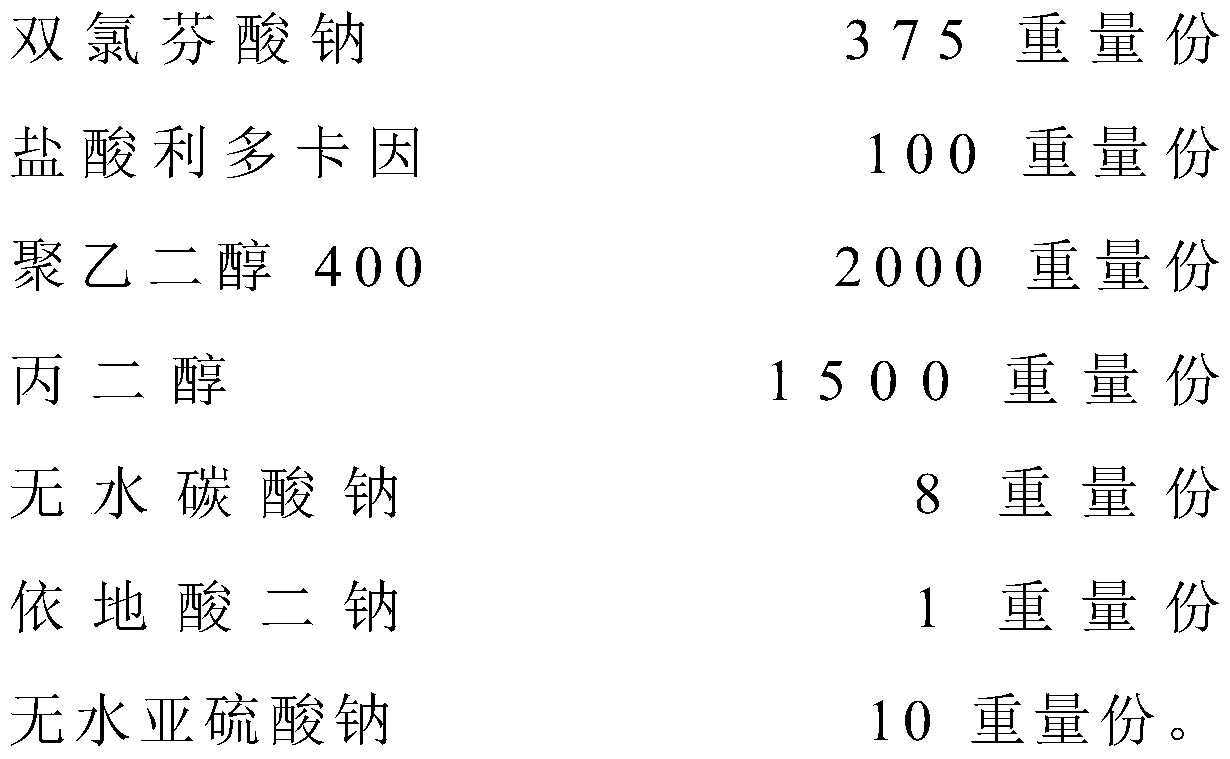
The invention relates to the technical field of red diclofenac sodium and lidocaine hydrochloride injections and preparation thereof, in particular to a diclofenac sodium and lidocaine hydrochloride injection and a preparation method thereof. The diclofenac sodium and lidocaine hydrochloride injection is prepared from the following raw materials in parts by weight: 300 to 420 parts of diclofenac sodium, 80 to 150 parts of lidocaine hydrochloride, 1200 to 2500 parts of polyethylene glycol 400, 1000 to 2000 parts of propylene glycol, 1-10 parts of anhydrous sodium carbonate, 0.5 to 1.5 parts ofedetate disodium, and 5-12 parts of anhydrous sodium sulfite. The anhydrous sodium carbonate is used as a pH stabilizer instead of the existing cysteine, thereby lowering the production cost and enhancing the economic benefit under the condition of not influencing the drug effect.
Owner:马鞍山思哲知识产权服务有限公司
Synthesis method of lidocaine hydrochloride
ActiveCN112441938AHigh yieldHigh purityOrganic compound preparationCarboxylic acid amides preparationLignocaine hydrochlorideDimethylaniline N-oxide
The invention provides a synthesis method for lidocaine hydrochloride. The synthesis method comprises the following steps: carrying out a heating reaction on methyl chloroacetate and 2,6-dimethylaniline in an acetonitrile solvent to obtain an intermediate 1; adding the intermediate 1, diethylamine and acetonitrile into a reaction kettle, carrying out a reaction, then carrying out post-treatment, and performing separating to obtain lidocaine alkali; adding lidocaine alkali and acetone into the reaction kettle, adding hydrochloric acid, and carrying out stirring and heating until the lidocaine alkali is dissolved; and then carrying out post-treatment such as decoloration and crystallization to obtain the lidocaine hydrochloride. According to the method, chloroacetyl chloride is replaced withmethyl chloroacetate, acetonitrile is selected as a reaction solvent, and through cooperation of all the conditions, a production environment is improved, safety in a production process is improved,and the yield and purity of lidocaine hydrochloride are remarkably improved.
Owner:常州康普药业有限公司
External gel for skin wounds, and preparation method of external gel
ActiveCN112121146APromote formationHas a preventive effectAntibacterial agentsHydroxy compound active ingredientsCentella asiatica extractCollagen fibres
The invention discloses an external gel for skin wounds. The external gel is prepared from the following raw materials in parts by weight: 1-3 parts of an active component A, 4-6 parts of an active component B, 10-14 parts of a Calendula arvensis extract, 16-20 parts of a Centella asiatica extract, and 60-80 parts of an auxiliary material, wherein the active component A is at least two of polymyxin B sulfate, neomycin sulfate and bacitracin, the active component B is at least one of lidocaine hydrochloride, pramoxine hydrochloride, procaine hydrochloride and menthol, and the auxiliary materialcomprises the following raw materials in parts by weight: 0.5-3 parts of carbomer, 40-75 parts of an organic solvent, and 20-50 parts of purified water. The external gel provided by the invention hasprevention and treatment effects on the bacterial infection of skin wound parts, has analgesic and hemostatic effects on skin wounds, can promote the formation of capillaries and collagenous fibers of wound surfaces, reduces the healing days of wound surfaces, and can be used for the healing of skin wounds such as burn wounds, incised wounds and scald wounds, the healed skin is smooth and free ofscars, and thereby the healing effect is good.
Owner:SHANDONG RUIAN PHARMA CO LTD
Compound lidocaine microneedle patch for easing pain
InactiveCN114869861AFacilitated releasePromote absorptionOrganic active ingredientsNervous disorderPolyvinyl alcoholPyrrolidinones
The invention discloses a compound lidocaine microneedle patch for analgesia, the microneedle patch comprises a needle tip layer and a backing layer, the needle tip layer is prepared from 1-5 parts by weight of lidocaine hydrochloride, 1-5 parts by weight of prilocaine hydrochloride and 90-98 parts by weight of a matrix material, the backing layer is prepared from 160-240 parts by weight of a backing material, and the backing layer is prepared from a matrix material. The matrix material is selected from one or more of hyaluronic acid, chondroitin sulfate and polyvinylpyrrolidone, the backing material is selected from one or more of hyaluronic acid, polyvinyl alcohol and glucan, and the traditional Chinese medicine composition has the advantages of being stable in quality, good in treatment effect and capable of achieving industrial production.
Owner:THE EYE HOSPITAL OF WENZHOU MEDICAL UNIV
Preparation process of sterilized lidocaine hydrochloride injection packaged by plastic ampoule
PendingCN114246958AImprove sealingEasy to store and transportOrganic active ingredientsInorganic non-active ingredientsLignocaine hydrochloridePolypropylene
The invention belongs to the field of pharmaceutical preparations, and particularly relates to research on a lidocaine hydrochloride sterilization process and a packing material. According to the lidocaine hydrochloride injection disclosed by the invention, the polypropylene plastic ampoule is used as a packing material of the lidocaine hydrochloride injection, and the sterilization process is that superheated water is sterilized for 15 minutes at 121 DEG C. The product quality is stable, the problems caused by the glass ampoule can be well solved, the clinical configuration process is optimized, and the lidocaine hydrochloride injection is convenient to transport and meets the environmental protection requirement.
Owner:NANJING GRITPHARMA CO LTD
Oral medical ice for preventing and treating oral ulcer and infection
InactiveCN105833250AShorten healing timeShorten the course of the diseaseOrganic active ingredientsPeptide/protein ingredientsArteriolar VasoconstrictionSide effect
The invention discloses oral medical ice for preventing and treating oral ulcer and infection. The oral medical ice comprises, by weight, 50-80 parts of Momorica dioica, 40-60 parts of Flos Rhododendri Anthopgonoidi, 40-70 parts of Flos Lonicerae, 10-40 parts of Fructus Forsythiae, 10-45 parts of Radix Ophiopogonis, 15-40 parts of Radix Scrophulariae, 5-20 parts of Rhizoma Corydalis, 5-15 parts of Radix Glycyrrhizae, granulocyte-macrophage colony stimulating factor, calcium folinate, lidocaine hydrochloride and vitamin C. Per 100ml of the oral medical ice contains 60-80microgram of the granulocyte-macrophage colony stimulating factor, 40-80mg of the calcium folinate, 300-400mg of the lidocaine hydrochloride and 400-500mg of the vitamin C. The oral medical ice has the advantages that pains can be alleviated for patients, vasoconstriction of oral mucosa is realized to reduce exudation, and accordingly oral hygiene is benefited; oral ulcer healing time can be shortened, a disease course is shortened, toxic and side effects are avoided, and high compliance is achieved; easiness in material acquisition, low cost and promising application prospect are realized.
Owner:杨力
Implant material capable of being used for human body soft tissue filling
ActiveCN113413484AGood biocompatibilityProlong degradation timeTissue regenerationProsthesisHuman bodyEngineering
The invention relates to the technical field of medical materials, in particular to an implant material capable of being used for human body soft tissue filling. In order to solve the problems that in the prior art, the degradation speed is high, displacement is likely to occur, the filling effect cannot be well kept, and repeated injection filling is needed, the implant material capable of being used for human body soft tissue filling is provided and comprises sodium hyaluronate, bioactive inorganic silicate, lidocaine hydrochloride and dexamethasone. Cross-linked sodium hyaluronate gel is used as an implantation carrier, bioactive inorganic silicate particle components are compounded, and medicines such as lidocaine hydrochloride and dexamethasone are matched and injected into collapsed soft tissues needing to be filled, so that the collapsed soft tissues of a patient are recovered; through the physical properties of the cross-linked sodium hyaluronate and the bioactive inorganic silicate particles and the excellent biocompatibility, the degradation time can be greatly prolonged and is 5-8 times of the degradation time of the sodium hyaluronate gel in the prior art, and the filling effect can be kept for a long time.
Owner:ZHEJIANG SUJIA MEDICAL DEVICE
A pharmaceutical composition for embolization therapy and pain relief and its preparation method
ActiveCN101716349BGive full play to the role of pain reliefHigh drug loadingAntipyreticAnalgesicsEmbolization TherapyDouble bond
The invention provides a medicine composite used for embolotherapy and acesodyne and a preparation method thereof. The medicine composite comprises a biocompatibility macromolecular compound containing hydroxy, a monomer containing unsaturated double bond and anion group, a polymer, and local anesthetic containing amino group, wherein the polymer is generated through a polymerization reaction of an optional vinyl monomer and the polymerization reaction is initiated by free radicals, and the local anesthetic is combined to an anion group of the generated polymer. In the invention, lidocaine hydrochloride is combined to a polymer carrier; which can give full play to the acesodyne effect of the local anesthetic in the embolotherapy; the anion part of the polymer can properly combine with the local anesthetic containing the amino group, which can both realize higher medicine loading capacity and enable the medicine in an emboliaztion agent to be exchanged by cations in vivo and then slowly released. Moreover, the polymer emboliaztion carrier has simple technology, low cost, and suitability for large scale industrial production.
Owner:HYGEA MEDICAL TECH CO LTD
Production method and application of lidocaine hydrochloride
PendingCN112375009ALow incidence of adverse reactionsImprove product qualityOrganic compound preparationCarboxylic acid amides preparationDimethylaniline N-oxideBiology
The invention discloses a production method and application of lidocaine hydrochloride, wherein the production method comprises the steps: carrying out acylation reaction on 2,6-dimethylaniline and chloroacetyl chloride to obtain a chloroacetyl compound, carrying out condensation reaction on the chloroacetyl compound and diethylamine to obtain lidocaine, and reacting lidocaine with hydrogen chloride gas to obtain a lidocaine hydrochloride crude product; refining the lidocaine hydrochloride crude product to obtain lidocaine hydrochloride, wherein solvents adopted in the acylation reaction and the condensation reaction are toluene, solvents of lidocaine and hydrogen chloride gas are acetone, and a refined solvent system is acetone and water; and after the condensation reaction, extracting the material with an acid solution, adding activated carbon for adsorption, filtering to remove the activated carbon, and adding an alkaline solution for treatment to obtain lidocaine. According to themethod, impurities of lidocaine hydrochloride can be greatly reduced, so that the safety and effectiveness of the lidocaine hydrochloride product are improved.
Owner:山东华鲁制药有限公司
A kind of radionuclide pollution decontamination agent and its preparation method and application
ActiveCN109700826BExtensive coordination abilityGood biocompatibilityOrganic active ingredientsAntinoxious agentsLignocaine hydrochloridePhosphate
A radionuclide contamination decontamination agent and its preparation method and application, the decontamination agent comprises the following components in mass percentage: EDTA·Na 2 : 1.0%‑20%, carboxymethyl chitosan: 0.1%‑0.4%, sodium alginate: 0.05%‑0.3%, lidocaine hydrochloride: 0.5%‑2.0%, the rest is pure water or phosphate buffer, The pH is 6.9‑7.0. It also provides its preparation method: accurately weigh EDTA‑Na in proportion 2 , carboxymethyl chitosan, sodium alginate and lidocaine hydrochloride, EDTA‑Na 2 Heat to dissolve under alkaline conditions, then dissolve carboxymethyl chitosan and sodium alginate, then mix the two, stir evenly, then add lidocaine hydrochloride solution, use HCl to adjust the pH to 6.7‑6.9, stir to dissolve ,Volume. The decontamination agent of the invention has the function of chelating and adsorbing various radionuclides, has no toxic and side effects, and can be used as a low-toxicity and high-efficiency radionuclide pollution decontamination agent.
Owner:中国人民解放军海军特色医学中心
Effective and safe injection for treating spontaneous pneumothorax
InactiveCN111840216AFast bondingReduced responseInorganic active ingredientsPharmaceutical delivery mechanismPneumothoraxThrombus
The invention discloses an effective and safe injection for treating spontaneous pneumothorax. The injection for treating spontaneous pneumothorax comprises alpha-n-butyl cyanoacrylate, nano silicon dioxide and a 50% glucose solution. The invention also provides an effective and safe injection for treating refractory pneumothorax. The injection for treating refractory pneumothorax comprises alpha-n-butyl cyanoacrylate, a human fibrin adhesive, 10g of talcum powder, a 50% glucose solution, normal saline and lidocaine hydrochloride. According to the invention, alpha-n-butyl cyanoacrylate is added, so that alpha-n-butyl cyanoacrylate can be used for quickly adhering body tissues at room temperature, and is strong in adhesion force and non-toxic; the reaction to tissues is small, thrombus is not formed, and simple sterilization can be realized; no trace is left after skin incision anastomosis, and the hemostatic function is achieved on a wound; the alpha-n-butyl cyanoacrylate can also be used for adhesion and hemostasis of visceral organs; and the adopted nano silicon dioxide has ultraviolet-resistant optical performance, so that the ageing resistance, strength and chemical resistanceof other materials can be improved.
Owner:AFFILIATED HOSPITAL OF ZUNYI MEDICAL COLLEGE
Drug addiction-stopping formulation and preparation thereof
InactiveCN100363006CEnsure safetyGuaranteed stabilityOrganic active ingredientsNervous disorderWithdrawal syndromeSodium acetate
The invention relates to a drug rehabilitation preparation and method for preparation, wherein the preparation comprises predetermined amount of tetrodontoxin monomer as the main effective composition, right amount of auxiliary material carrier, function modifier and tetrodontoxin derivative as the stabilizer, the acidic dissolvent carrier is selected from acetic acid / sodium acetate, or citric acid / sodium citrate, or citric acid / disodium hydrogen phosphate, the function modifier is at least one selected from trichlorbutanolum, benzoic alcohol and lignocaine hydrochloride.
Owner:XIAMEN ZHAOYANG BIOLOGICAL ENG +1
Compound lidocaine gel used for pets, and preparation method and quality control method thereof
InactiveCN112220789AFill the gaps in medicationStable and controllable qualityAntibacterial agentsOrganic active ingredientsDiseasePeripheral neuron
The invention relates to the field of pharmaceutical preparations, in particular to a compound lidocaine gel used for surface anesthesia and bacteria resistance of pets, and a preparation method and quality control method thereof. The compound lidocaine gel disclosed by the invention comprises the following components: lidocaine hydrochloride, antibiotics, a gel matrix and a transdermal enhancer;the antibiotics are selected from one of or a mixture of two or more of enrofloxacin, ofloxacin and pefloxacin mesylate. The compound lidocaine gel disclosed by the invention is used for laryngeal lubrication and peripheral nerve anesthesia during tracheal intubation; when a catheter and a nasal feeding tube are inserted, the compound lidocaine gel is used for mucous membrane lubrication and anesthesia to reduce the discomfort and mucous membrane hyperemia of an animal; For a severe anal gland disease, when the anal gland needs to be manually extruded, the compound lidocaine gel is used for rectum lubrication and local anesthesia; and the compound lidocaine gel is used for surface anesthesia of part of ophthalmologic operations, and the gel can maintain local tissues moist in comparison with an injection.
Owner:马保臣
Compound lidocaine nano liposome gel and preparation method thereof
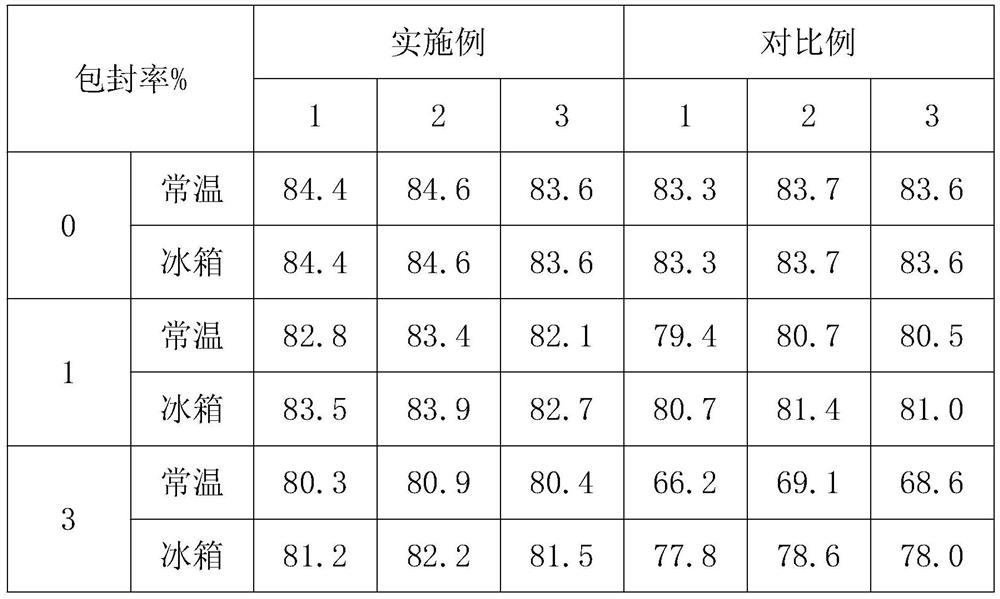
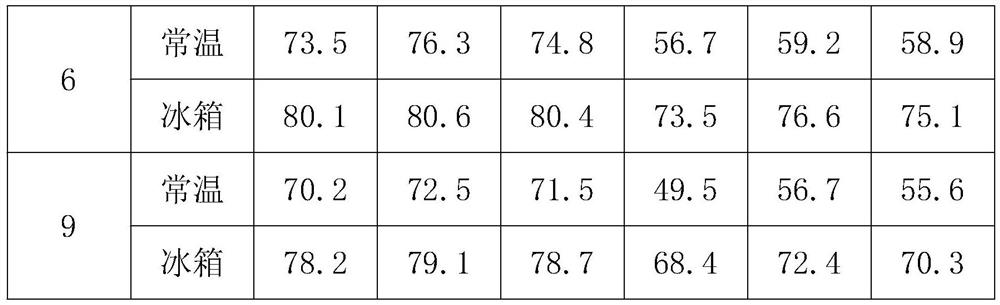
PendingCN112353803AReasonable formulaGood stability of encapsulation rateOrganic active ingredientsAerosol deliveryCelluloseCholesterol
The invention provides compound lidocaine nano-liposome gel and a preparation method thereof. The nano-liposome gel is prepared from the following raw materials: lidocaine hydrochloride, tetracaine hydrochloride, tetracaine base, lidocaine base, dyclonine hydrochloride, procaine hydrochloride, soyabean lecithin, cholesterol, xanthan gum, carbomer and sodium carboxymethyl cellulose. The preparationmethod of the nano-liposome gel comprises the following steps: S1, preparing raw materials; s2, mixing soyabean lecithin and cholesterol to obtain a mixture A; s3, dissolving carbomer to obtain a mixture B; and S4, adding lidocaine hydrochloride, tetracaine hydrochloride, dyclonine hydrochloride, procaine hydrochloride and the mixture B into the mixture A, performing stirring, adding tetracaine alkali and lidocaine alkali, performing cooling, adding xanthan gum and sodium carboxymethyl cellulose, performing stirring, and performing homogenizing to obtain the compound lidocaine nano-liposome gel. The nano-liposome gel provided by the invention is good in stability in a normal-temperature state and a low-temperature state.
Owner:中国人民解放军第三〇五医院
Process for obtaining heat sterilizable injectable hydrogels based on hyaluronic acid containing lidocaine and alkaline agents added in powder form
ActiveCN105705137BOrganic active ingredientsCosmetic preparationsLignocaine hydrochlorideUronic acid
Owner:ANTEIS SA
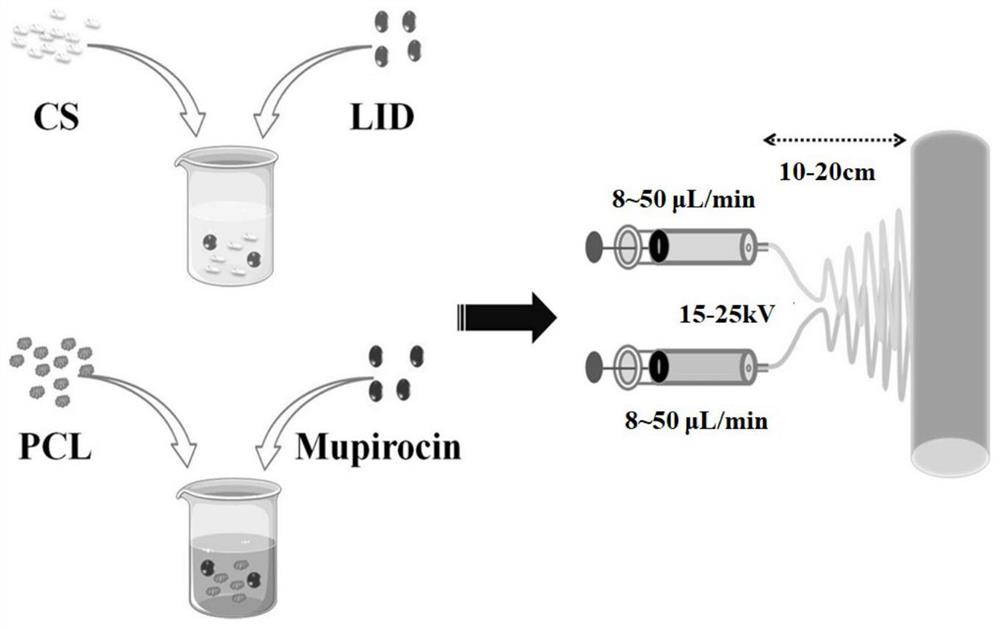
Chitosan/polycaprolactone composite nanofiber membrane material and application thereof
InactiveCN111793900AAchieve aggregationAchieve healingPharmaceutical delivery mechanismFilament/thread formingFiberAntibacterial activity
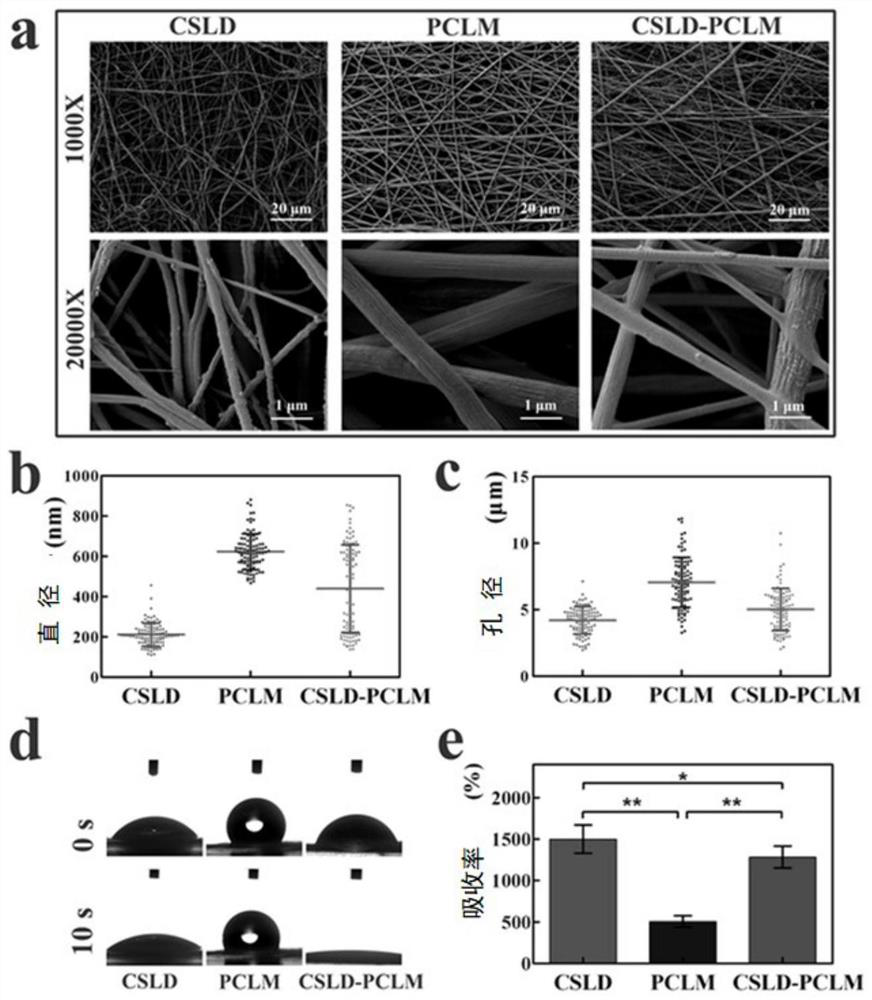
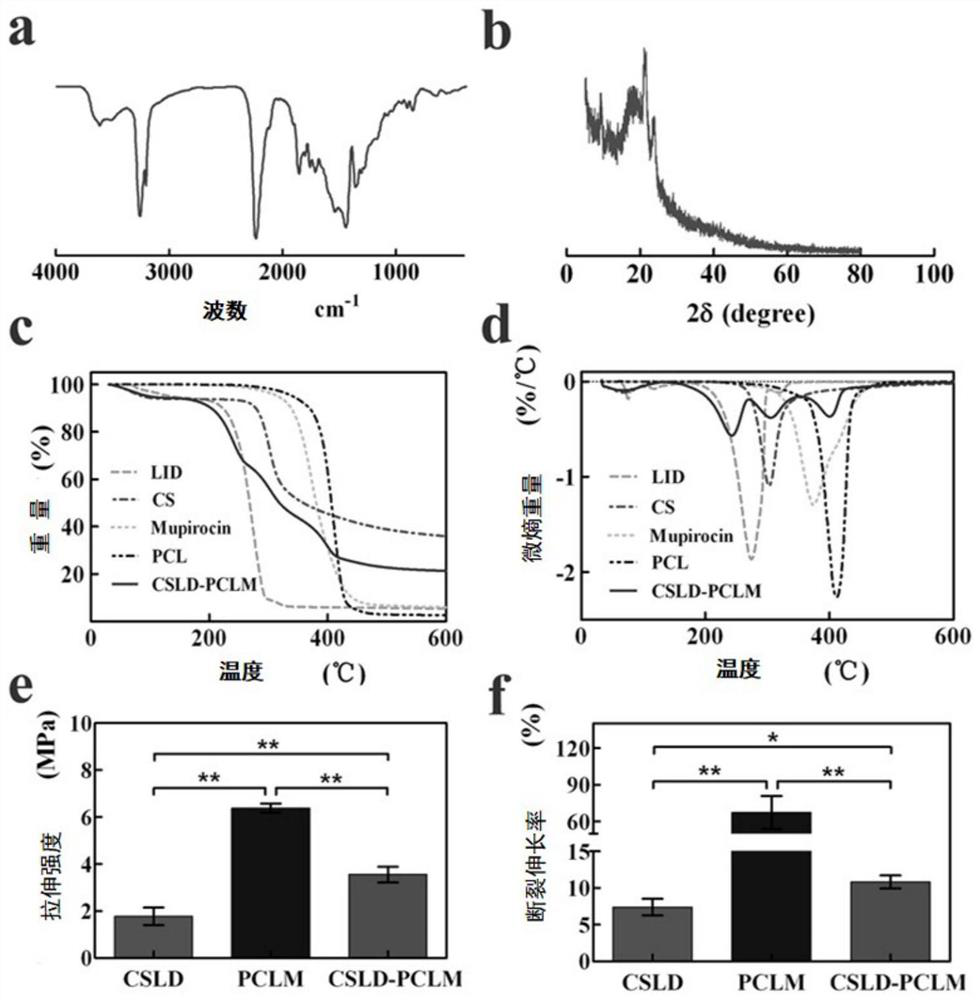
The invention discloses a chitosan / polycaprolactone composite nanofiber membrane material. The chitosan / polycaprolactone composite nanofiber membrane material is prepared by the following steps that a6-15wt% polycaprolactone solution is prepared, and 1-4wt% mupirocin is added; a 5-12wt% chitosan solution is prepared, and 0.5-8wt% lidocaine hydrochloride is added; the solutions are put into a feeding device of electrospinning equipment, the feeding rate is 8-50[mu]L / min, the receiving distance is 10-20cm, and the rotating speed of a receiver is 100-250 rpm / min; a high-voltage power supply is turned on, a voltage of 15-25 kV is applied, and the solutions are sprayed onto the fiber receiver for forming; and drying is carried out to remove the solvent, so that a composite nanofiber membrane is obtained. The composite nanofiber membrane exhibits excellent hydrophilicity, swelling rate, absorption capacity, thermal stability, mechanical properties and effective blood clotting ability, and further exhibits excellent cell compatibility and antibacterial activity; different release curves of different functional drugs can be realized; and the wound healing process can be significantly enhanced, and a synergistic effect of wound healing is achieved.
Owner:中国人民解放军陆军特色医学中心
Compound Chinese and western medicine mixture for children stomatitis and preparation method thereof
InactiveCN100536887CQuick clearGood curative effectHeavy metal active ingredientsDigestive systemAngelica dahuricaQingdai
The invention relates to the field of Chinese and Western medicine compound preparations, and specifically discloses a compound Chinese and Western medicine mixture for children with stomatitis and a preparation method thereof. Its prescription is composed of the following ingredients: flea 2g-4g, shegan 3g-5g, white fresh skin 3g-5g, angelica dahurica 0.5g-2g, Qingdai 0.5g-2g, root ginseng 0.3g-3g, borax 0.5g g-3g, cinnabar 0.05g-1g, borneol 0.02g-2g, gentamicin 80,000 units-160,000 units, lidocaine hydrochloride 0.05g-0.1g. Its preparation method is: according to the proportion of the above-mentioned Chinese medicine components, select pure and high-quality herbal medicine decoction pieces, remove impurities, first grind the other eight Chinese medicine raw materials except cinnabar into 120-160 mesh fine powder, and then grind the cinnabar to a fineness of 120-160 mesh No friction is the degree, dry it into a block, then mix the ground cinnabar with the previously developed Chinese medicine ingredients evenly, put it in a light-proof sealed container, and add gentamicin according to the proportion in the formula when it is used Sodium glutamate, lidocaine hydrochloride and an appropriate amount of 0.9% physiological saline are mixed uniformly to make an external water agent that can be sprayed. The present invention has high curative effect and short course of treatment, is an external spraying medicament, can be sprayed, is convenient for children to use, and has obvious advantages compared with existing medicines and treatments.
Owner:苏小旦
Compound lidocaine gel patch
ActiveCN113398101AFacilitated releasePromote absorptionOrganic active ingredientsNervous disorderLignocaine hydrochlorideAdhesive
The invention relates to a compound lidocaine / prilocaine gel patch. The gel patch is prepared from following components in percentage by weight: 4%-8% of lidocaine hydrochloride, 4%-8% of prilocaine hydrochloride, 2%-5% of a framework material, 5%-10% of a filler, 20%-30% of an adhesive, 3%-9% of a tackifier, 1%-5% of a cross-linking agent, 2%-4% of a transdermal absorption enhancer, 30%-50% of a humectant, 0.005%-0.02% of a preservative, 0.005%-0.02% of a complexing agent and 0.5%-2% of a stabilizer. The compound lidocaine / prilocaine gel patch is stable in quality, good in treatment effect, simple in preparation process and capable of realizing industrial production.
Owner:THE EYE HOSPITAL OF WENZHOU MEDICAL UNIV
Lidocaine hydrochloride gel and preparation method thereof
ActiveCN113244167AHas a caustic effectGood transdermal effectOrganic active ingredientsAerosol deliveryLignocaine hydrochlorideActive agent
The invention relates to the technical field of pharmaceutical preparations, and particularly discloses lidocaine hydrochloride gel and a preparation method thereof. The gel is prepared from the following components in percentage by mass of 11 percent to 13 percent of poloxamer 407, 0.1 percent to 0.5 percent of poloxamer 188 and 86.5 percent to 88.5 percent of nanoemulsion, wherein the nanoemulsion is prepared from the following components in percentage by mass of 2 percent to 5 percent of an oil phase, 15 percent to 23 percent of a surfactant, 5 percent to 10 percent of a cosurfactant, 60 percent to 70 percent of deionized water and 2 percent of lidocaine hydrochloride on the basis that the total amount of the components is 100 percent. According to the gel, lidocaine hydrochloride is dispersed in the nanoemulsion, a gel matrix with a temperature-sensitive characteristic is formed by poloxamer 407 and poloxamer 188 according to a specific ratio, and the nanoemulsion is loaded on the gel matrix to form the nano-emulsion-in-situ gel which has a long-acting analgesic effect.
Owner:HEBEI UNIVERSITY OF SCIENCE AND TECHNOLOGY
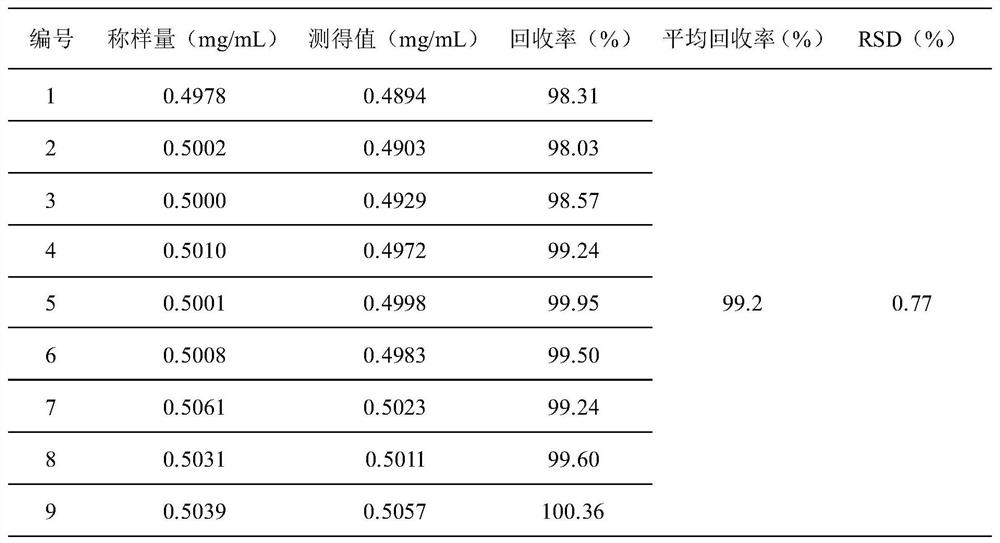
HPLC detection method for degradation impurities in lidocaine hydrochloride and preparation thereof
The invention discloses an HPLC detection method for degradation impurities in lidocaine hydrochloride and a preparation thereof. The method comprises the steps of separating and determining the degradation impurities in lidocaine hydrochloride and the preparation thereof by using a high performance liquid chromatograph and adopting an isocratic elution method, wherein a chromatographic column with the detection wavelength of 230+ / -2nm takes octadecylsilane bonded silica gel as a filler, a mixed solution composed of a 0.01 mol / L phosphate buffer solution with a volume percentage of 50 to 60% and an organic phase with a volume percentage of 40 to 50% is used as a mobile phase, and the pH value of the organic phase is adjusted to 8.0 with phosphoric acid. The method disclosed by the invention has the characteristics of good separation degree, simplicity, rapidness, strong specificity and high sensitivity, and can be used for carrying out quality control on degradation impurities possibly introduced in production and storage of lidocaine hydrochloride and the preparation thereof, so that the quality of the whole product and the clinical medication safety are ensured.
Owner:TIANSHENG PHARMA GROUP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com