Diclofenac sodium and lidocaine hydrochloride injection and preparation method thereof
A technology of lidocaine hydrochloride and diclofenac sodium, applied in the field of diclofenac sodium lidocaine hydrochloride injection and preparation thereof, can solve the problem of high cost of cysteine, and achieve the effects of reducing production cost and improving economic benefits
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0025] Under preferred conditions, the preparation method of described diclofenac sodium lidocaine hydrochloride injection comprises the steps:
[0026] (1) Preparation of medicinal solution A: Dissolve anhydrous sodium sulfite in 30-40vol% water for injection, then add propylene glycol and mix well, then add polyethylene glycol 400 and mix well, then slowly add diclofenac sodium, stir to make it Dissolve to obtain liquid A;
[0027] (2) Medicinal solution B: dissolving lidocaine hydrochloride and edetate disodium in 15-20 vol% water for injection to obtain medicinal solution B;
[0028] (3) Under the condition of stirring, slowly add medicinal solution B to medicinal solution A, and keep stirring, adjust the pH of the system to 7.5-8.5 with sodium carbonate solution after mixing, add the remaining amount of water for injection after cooling to room temperature, Stir, let stand for 15 minutes, and control the pH of the system to 7.5-8.5;
[0029] (4) Coarse filtration: use a...
Embodiment 1
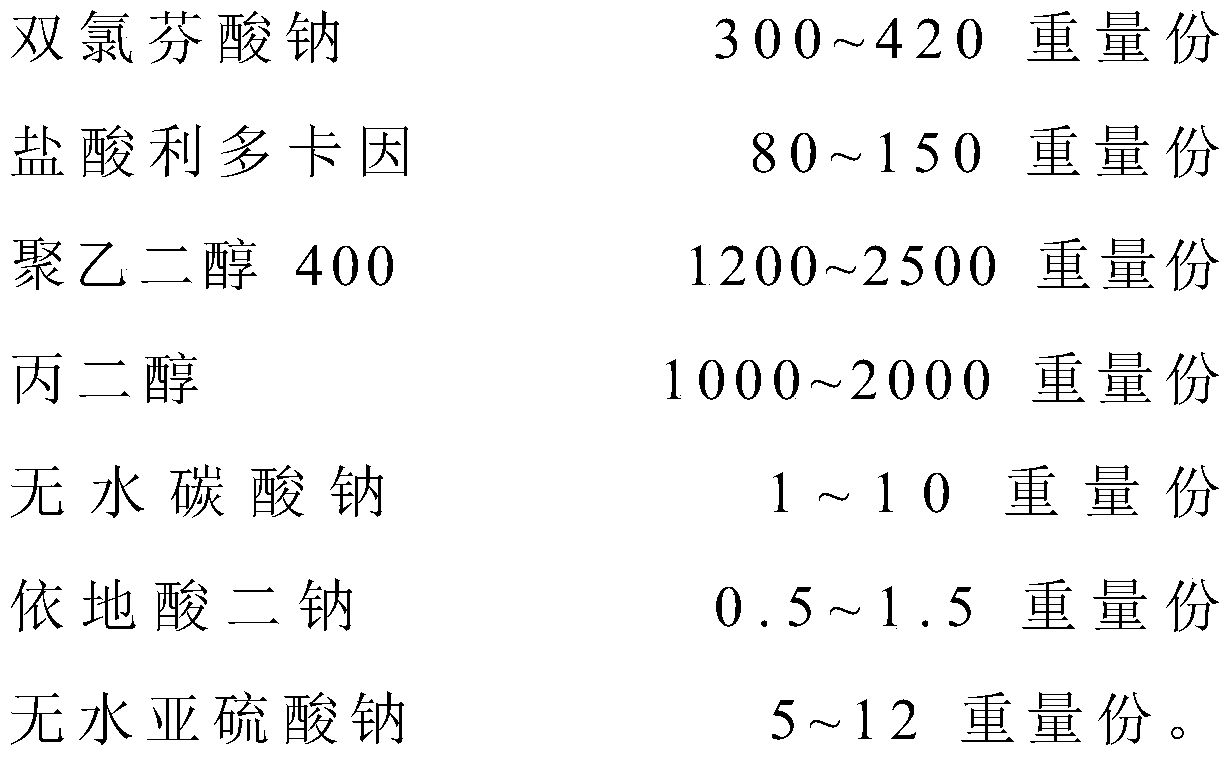
[0038] A diclofenac sodium lidocaine hydrochloride injection is made from the following raw materials in parts by weight:
[0039]
[0040] The preparation method of described diclofenac sodium lidocaine hydrochloride injection comprises the steps:
[0041] (1) Preparation of medicinal solution A: Dissolve anhydrous sodium sulfite in 33.3vol% water for injection, then add propylene glycol and mix well, then add polyethylene glycol 400 and mix well, then slowly add diclofenac sodium, stir to dissolve , to obtain liquid A;
[0042] (2) Medicinal solution B: dissolve lidocaine hydrochloride and edetate disodium in 16.6vol% water for injection to obtain medicinal solution B;
[0043] (3) Under the condition of stirring, slowly add the medicinal solution B to the medicinal solution A, and keep stirring, after mixing, adjust the pH of the system to 7.75-8.25 with a sodium carbonate solution with a mass fraction of 15wt%, cool to room temperature and then add 50.2vol% water for ...
Embodiment 2
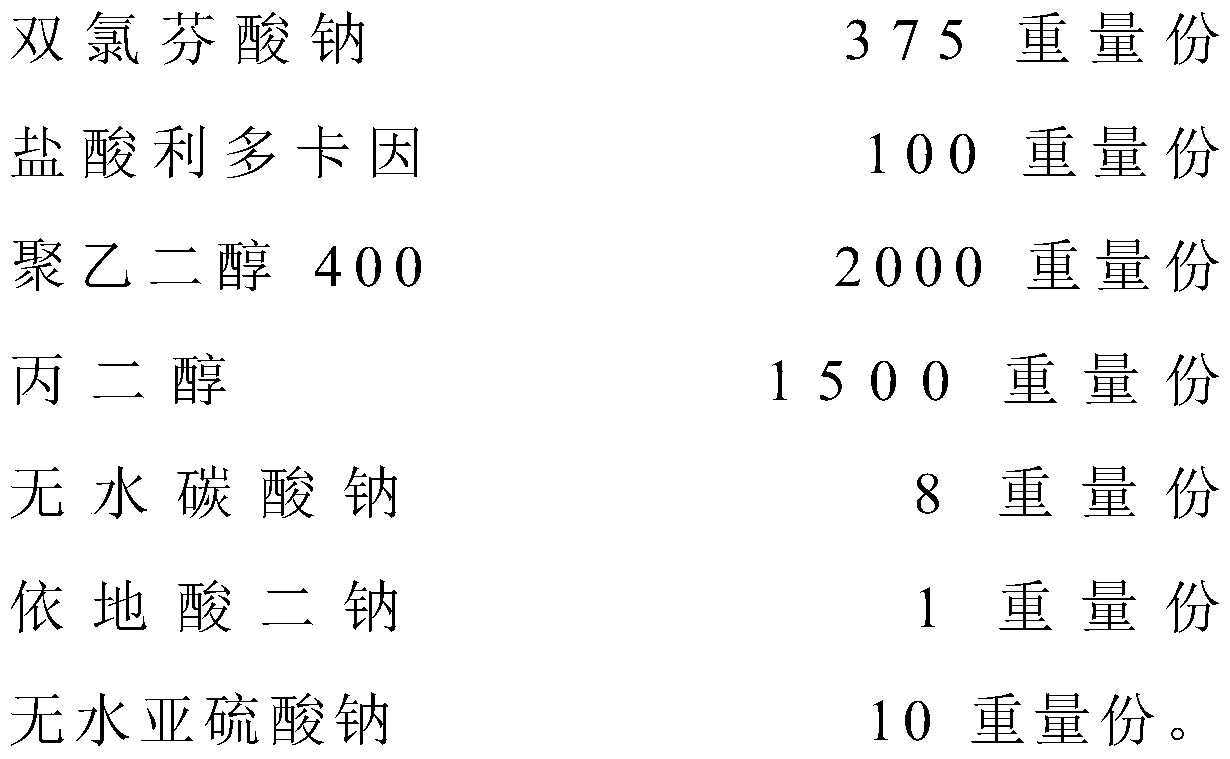
[0047] A diclofenac sodium lidocaine hydrochloride injection is made from the following raw materials in parts by weight:
[0048]
[0049] The preparation method of described diclofenac sodium lidocaine hydrochloride injection comprises the steps:
[0050] (1) Preparation of medicinal solution A: Dissolve anhydrous sodium sulfite in 30vol% water for injection, then add propylene glycol and mix well, then add polyethylene glycol 400 and mix well, then slowly add diclofenac sodium, stir to dissolve, Get liquid A;
[0051] (2) Medicinal solution B: dissolve lidocaine hydrochloride and edetate disodium in 15vol% water for injection to obtain medicinal solution B;
[0052] (3) Under the condition of stirring, slowly add the medicinal solution B to the medicinal solution A, and keep stirring, after mixing, adjust the pH of the system to 7.5-8 with a sodium carbonate solution with a mass fraction of 12wt%, and add it after cooling to room temperature 55vol% water for injection,...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com