Production method and application of lidocaine hydrochloride
A technology for lidocaine hydrochloride and a production method, which is applied in chemical instruments and methods, preparation of carboxylic acid amides, preparation of organic compounds, etc., can solve the problems of no curative effect, adverse reactions, and excessive carcinogen isopropylidene ketone. , to achieve the effect of reducing the incidence and improving product quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
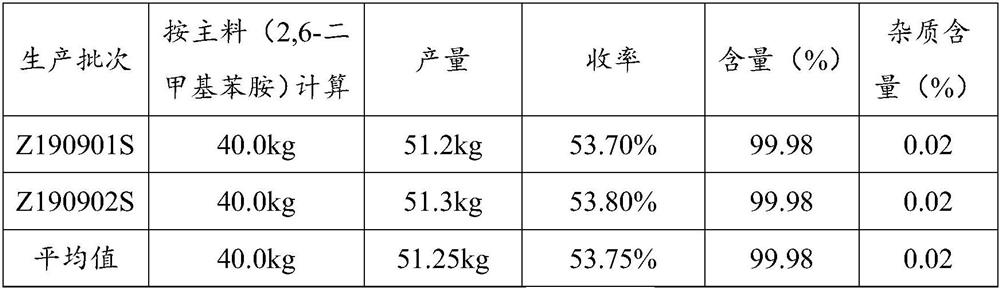
Embodiment 1
[0048] Acylation reaction
[0049] 2,6-Dimethylaniline (kg): Chloroacetyl chloride (kg): Toluene (kg) = 1: 1.25: 5
[0050] Add toluene and 2,6-dimethylaniline into the reaction tank. Chloroacetyl chloride was added dropwise at 10°C for 1 hour. After the drop was completed, it took 2 hours to raise the temperature to boiling; slowly add 2,6-dimethylaniline 1 times purified water dropwise. The lower aqueous acid layer was discarded. Then use 2wt% potassium carbonate solution to neutralize the remaining acid, test the pH of the water layer to be greater than 7, and let off the water layer. Lower the temperature, drop the temperature of the feed liquid to room temperature, and centrifugally filter to obtain white needle-like crystals, ie, chloroacetylate.
[0051] condensation reaction
[0052] Chloroacetylate (kg): diethylamine (kg): toluene (kg) = 1:0.6:11
[0053] Pump the toluene into the condensation tank, put in the chloroacetylate, suck in the diethylamine, start stirri...
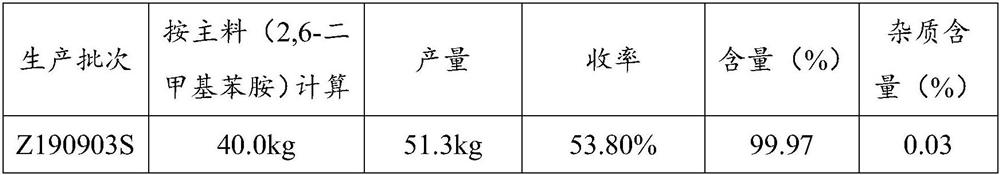
Embodiment 2
[0076] Acylation reaction
[0077] 2,6-Dimethylaniline (kg): Chloroacetyl chloride (kg): Toluene (kg) = 1:1:7
[0078] Add toluene and 2,6-dimethylaniline into the reaction tank. Chloroacetyl chloride was added dropwise at 20 o'clock for 1 hour. After the drop, it took 5 hours to raise the temperature to boiling; slowly add 2,6-dimethylaniline 2 times purified water dropwise. The lower aqueous acid layer was discarded. Then use 3wt% potassium carbonate solution to neutralize the remaining acid, test the pH of the water layer to be greater than 7, and let off the water layer. Lower the temperature, drop the temperature of the feed solution to room temperature, and centrifugally filter to obtain white needle-like crystals, namely chloroacetylated compounds.
[0079] condensation reaction
[0080] Chloroacetylate (kg): diethylamine (kg): toluene (kg) = 1:1:8
[0081] Pump the toluene into the condensation tank, put in the chloroacetylate, suck in the diethylamine, start stir...
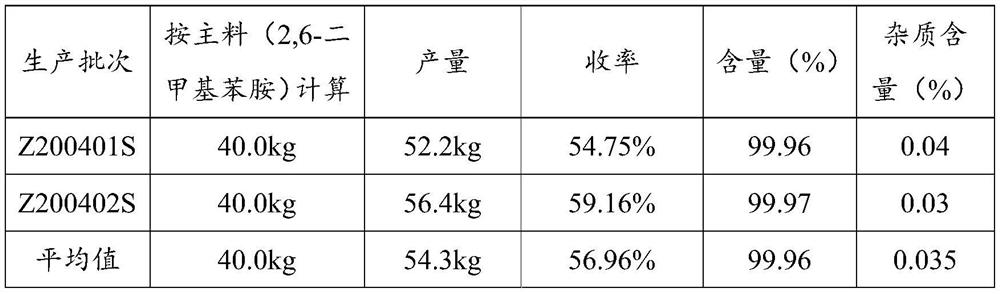
Embodiment 3
[0101] Acylation reaction
[0102] 2,6-Dimethylaniline (kg): Chloroacetyl chloride (kg): Toluene (kg) = 1:1.5:12
[0103] Add toluene and 2,6-dimethylaniline into the reaction tank. Chloroacetyl chloride was added dropwise at 50°C for 1 hour. After the drop, it took 7 hours to raise the temperature to boiling; slowly add 2,6-dimethylaniline 5 times purified water dropwise. The lower aqueous acid layer was discarded. Then use 5wt% potassium carbonate solution to neutralize the remaining acid, test the pH of the water layer to be greater than 7, and let off the water layer. Lower the temperature, drop the temperature of the feed solution to room temperature, and centrifugally filter to obtain white needle-like crystals, namely chloroacetylated compounds.
[0104] condensation reaction
[0105] Chloroacetylate (kg): diethylamine (kg): toluene (kg) = 1:1.5:5
[0106] Pump the toluene into the condensation tank, put in the chloroacetylate, suck in the diethylamine, start stirr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com