Implant material capable of being used for human body soft tissue filling
A technology for implanting materials and soft tissues, which is applied in the field of medical materials to achieve the effect of maintaining the filling effect, the raw materials are easily available, and the raw materials are simple
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
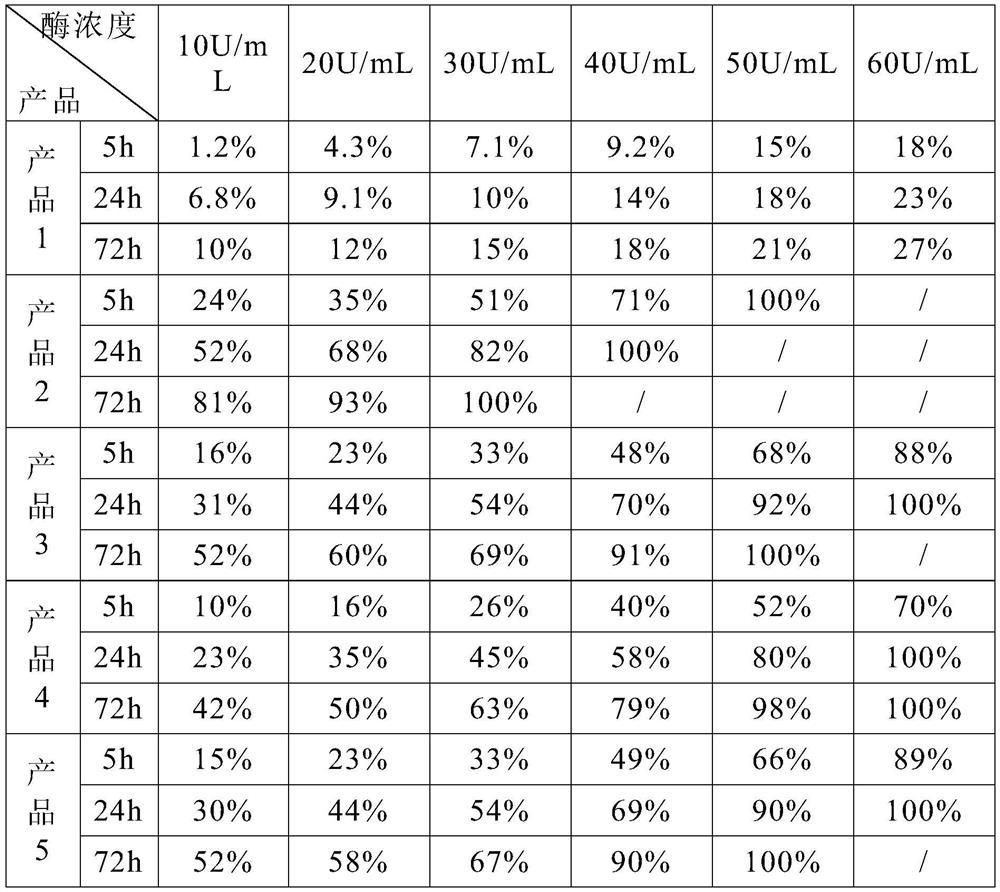
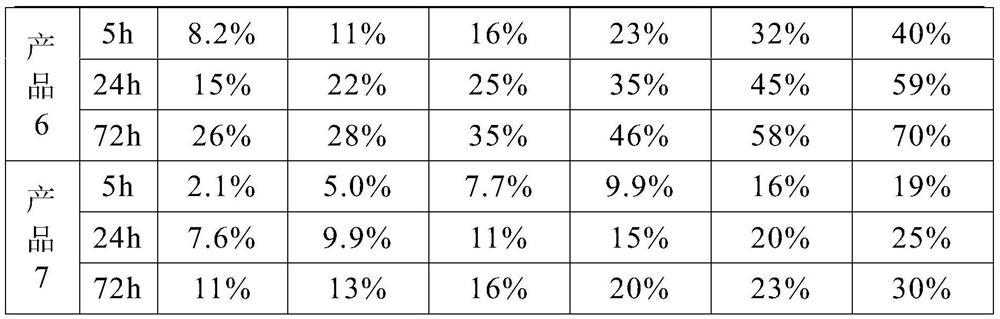
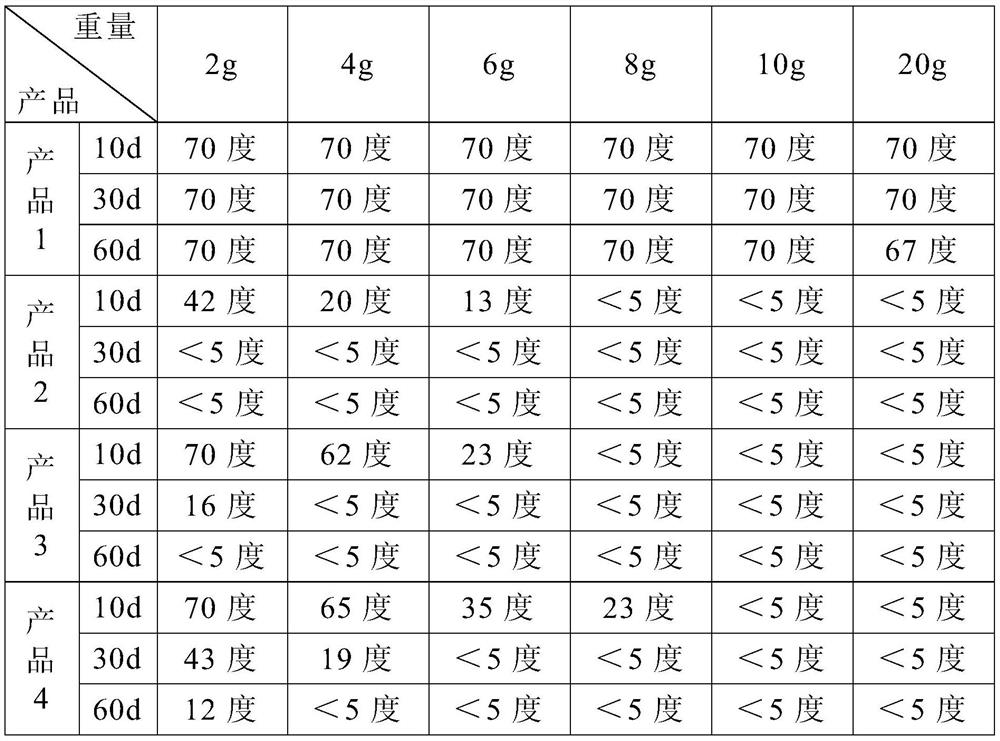
[0022] This embodiment provides an implant material that can be used for human soft tissue filling, including 50 parts by mass of cross-linked sodium hyaluronate gel, 14 parts of silicon dioxide, 8 parts of sodium oxide, 5 parts by mass 1 part of calcium oxide, 1 part of phosphorus pentoxide, 0.3 part of lidocaine hydrochloride and 0.75 part of dexamethasone.
[0023] Among them, the cross-linked sodium hyaluronate gel is prepared by the following method: according to the ratio of sodium hyaluronate powder and saline solution of 1:18, it is dissolved into a sodium hyaluronate solution, and sodium hydroxide is added to adjust the pH value of the solution to 9. , then add the cross-linking agent 1,4 butanediol diglycidyl ether, the amount of the cross-linking agent added is 3% of the mass of the sodium hyaluronate solution, slowly stir and heat to 70°C, cool to room temperature after continuing for 8 hours, At this time, the sodium hyaluronate solution has been cross-linked into...
Embodiment 2
[0026] This embodiment provides an implant material that can be used for human soft tissue filling, including 20 parts by mass of cross-linked sodium hyaluronate gel, 24 parts of silicon dioxide, 13 parts of sodium oxide, 18 parts by mass. 3 parts of calcium oxide, 4 parts of phosphorus pentoxide, 3 parts of lidocaine hydrochloride and 3 parts of dexamethasone.
[0027] Among them, the cross-linked sodium hyaluronate gel is prepared by the following method: dissolving sodium hyaluronate solution according to the ratio of sodium hyaluronate powder and normal saline 1:20, adding potassium hydroxide to adjust the pH value of the solution to 10 , then add the cross-linking agent divinyl sulfone, the amount of the cross-linking agent added is 5% of the mass of the sodium hyaluronate solution, slowly stir and heat to 80 ° C, cool to room temperature after continuing for 12 hours, at this time the sodium hyaluronate solution It has been cross-linked into a gel state, then add phospha...
Embodiment 3
[0030] This embodiment provides an implant material that can be used for human soft tissue filling, including 35 parts by mass of cross-linked sodium hyaluronate gel, 19 parts of silicon dioxide, 10 parts of sodium oxide, 12 parts by mass Parts of calcium oxide, 2.5 parts of phosphorus pentoxide, 1.5 parts of lidocaine hydrochloride and 2 parts of dexamethasone.
[0031] The preparation method of cross-linked sodium hyaluronate gel and biologically active inorganic silicate is the same as that in Example 1, so it will not be repeated here.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com