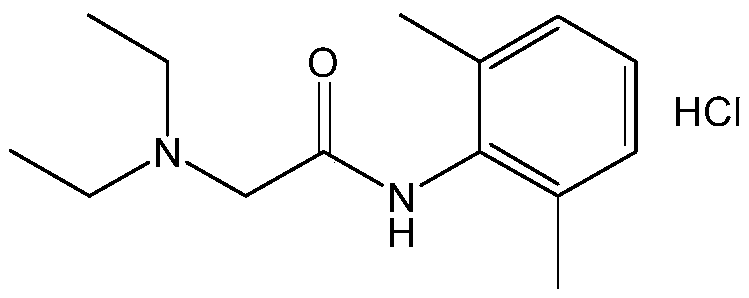
Preparation method of lidocaine hydrochloride
A technology of lidocaine hydrochloride and acid-binding agent, which is applied in the field of preparation of lidocaine hydrochloride, can solve the problems of cumbersome operation, low product yield, and difficulty in obtaining, etc., and achieves the method with simple procedures, avoiding multiple purifications, and easy The effect of control
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] 本实施例提供了一种盐酸利多卡因的制备方法,具体为:
[0021] (1)向500L搪瓷反应釜中加入丙酮200L、2,6-二甲基苯胺50.0kg(412.6mol)、碳酸钾142.6kg(1031.5mol),搅拌并调节物料温度至20±5℃,保持在此温度范围内高位滴加氯乙酰氯48.9kg(433.2mol),滴完后在此温度下搅拌反应0.5h。加入二乙胺33.2kg(453.9mol),加热至60±5℃反应8h,过滤,搅拌下向滤液中加入盐酸,调节滤液pH至4以下。
[0022] (2)将上述反应液在50±5℃下减压浓缩至无液体蒸出,加入200L丙酮,加热溶解完全后加入2kg活性炭,在55~65℃下搅拌脱色0.5h,压滤至洁净区结晶釜,搅拌降温结晶,降温至-5℃以下保持3h,甩滤,双锥40~50℃真空干燥4h,得白色结晶性粉末93.4kg,收率:78.38%,HPLC归一化纯度:99.84%。
Embodiment 2
[0024] 本实施例提供了一种盐酸利多卡因的制备方法,具体为:
[0025] (1)向500L搪瓷反应釜中加入丙酮200L、2,6-二甲基苯胺50.0kg(412.6mol)、碳酸钾114.1kg(825.2mol),搅拌并调节物料温度至20±5℃,保持在此温度范围内高位滴加氯乙酰氯41.9kg(371.3mol),滴完后在此温度下搅拌反应0.5h。加入二乙胺30.2kg(412.6mol),加热至60±5℃反应8h,过滤,搅拌下向滤液中加入盐酸,调节滤液pH至4以下。
[0026] (2)将上述反应液在50±5℃下减压浓缩至无液体蒸出,加入200L丙酮,加热溶解完全后加入2kg活性炭,在55~65℃下搅拌脱色0.5h,压滤至洁净区结晶釜,搅拌降温结晶,降温至-5℃以下保持3h,甩滤,双锥40~50℃真空干燥4h,得白色结晶性粉末85.2kg,收率:71.46%,HPLC归一化纯度:99.28%。
Embodiment 3
[0028] 本实施例提供了一种盐酸利多卡因的制备方法,具体为:
[0029] (1)向500L搪瓷反应釜中加入丙酮200L、2,6-二甲基苯胺50.0kg(412.6mol)、碳酸钾228.1kg(1650.4mol),搅拌并调节物料温度至20±5℃,保持在此温度范围内高位滴加氯乙酰氯55.9kg(495.1mol),滴完后在此温度下搅拌反应0.5h。加入二乙胺45.3kg(618.9mol),加热至60±5℃反应8h,过滤,搅拌下向滤液中加入盐酸,调节滤液pH至4以下。
[0030] (2)将上述反应液在50±5℃下减压浓缩至无液体蒸出,加入200L丙酮,加热溶解完全后加入2kg活性炭,在55~65℃下搅拌脱色0.5h,压滤至洁净区结晶釜,搅拌降温结晶,降温至-5℃以下保持3h,甩滤,双锥40~50℃真空干燥4h,得白色结晶性粉末88.4kg,收率:74.21%,HPLC归一化纯度:99.52%。
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com