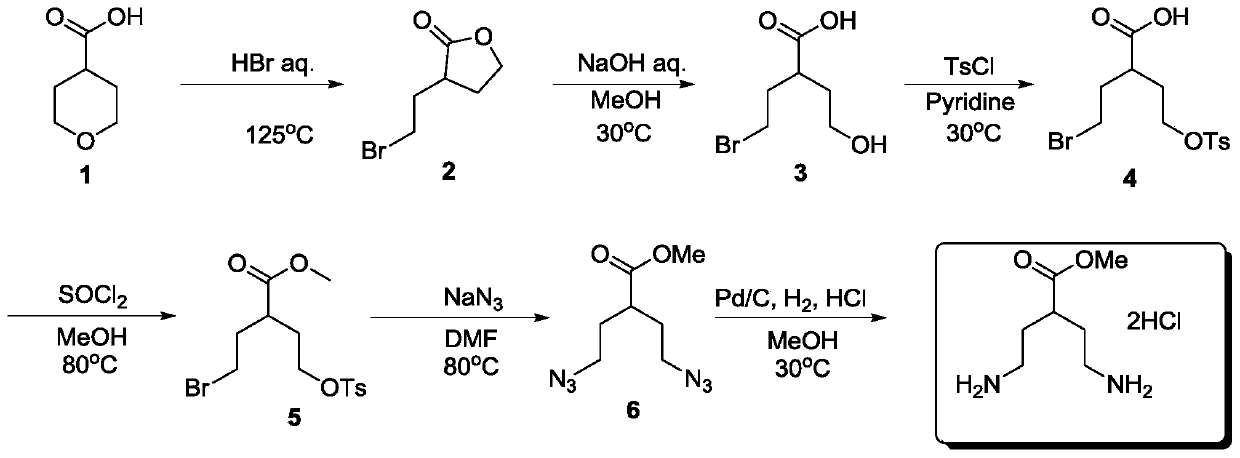
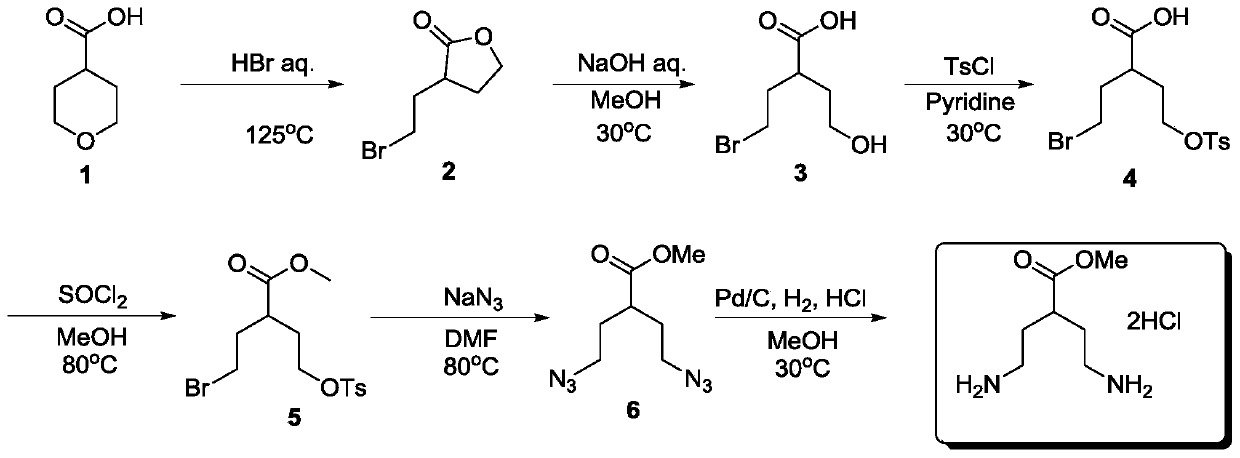
A kind of preparation method of methyl 4-amino-2-(2-ethylamino)butyrate dihydrochloride
A technology of methyl butyrate and dihydrochloride is applied in the preparation of sulfonic acid esters, the preparation of carboxylic acid esters/lactones, the preparation of organic compounds, etc., and can solve the problems of not getting the target product, affecting the yield and the like, To achieve the effect of good reaction selectivity, easy operation and few by-products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] A preparation method of 4-amino-2-(2-ethylamino) methyl butyrate dihydrochloride, comprising the steps:
[0025] (1) Preparation of 3-(2-bromoethyl)-dihydro-2(3H)-furanone
[0026] Add hydrobromic acid (1450g, 7.25mol, 6.6eq) into a 5-liter three-neck flask at room temperature, add tetrahydropyran-4-carboxylic acid (138.0g, 1.1mol, 1eq) under stirring, heat to 80°C and stir for 12h , TLC monitors raw material after reaction, adds 3000 milliliters of water to reaction solution and extracts with ethyl acetate three times (3 * 1000mL), combines organic layer, washes with water, washes with brine, sodium sulfate is dried, filters, and filtrate is spin-dried to obtain yellow oil 187 g was directly put into the next step without purification, and the HNMR purity was 95%.
[0027] 1 H NMR (400MHz, CDCl 3 )δ4.40(td, J=8.9,2.1Hz,1H),4.23(ddd,J=10.3,9.2,6.4Hz,1H),3.72–3.62(m,1H),3.49(ddd,J=10.3, 8.0, 5.6Hz, 1H), 2.91–2.75 (m, 1H), 2.47 (dddd, J=18.0, 8.1, 6.1, 3.0Hz, 2H), 2.0...
Embodiment 2
[0043] A preparation method of 4-amino-2-(2-ethylamino) methyl butyrate dihydrochloride, comprising the steps:
[0044] (1) Preparation of 3-(2-bromoethyl)-dihydro-2(3H)-furanone
[0045] Add hydrobromic acid (220g, 1.1mol, 1eq) to a 5-liter three-necked flask at room temperature, add tetrahydropyran-4-carboxylic acid (138.0g, 1.1mol, 1eq) under stirring, heat to reflux and stir for 6h, TLC After monitoring the reaction of raw materials, add 3000 milliliters of water to the reaction solution and extract three times with ethyl acetate (3 × 1000 mL), combine the organic layers, wash with water, wash with brine, dry over sodium sulfate, filter, spin the filtrate to obtain 190 g of yellow oil, It was directly put into the next step without purification, and the purity by HNMR was 95%.
[0046] 1 H NMR (400MHz, CDCl 3 )δ4.40(td, J=8.9,2.1Hz,1H),4.23(ddd,J=10.3,9.2,6.4Hz,1H),3.72–3.62(m,1H),3.49(ddd,J=10.3, 8.0, 5.6Hz, 1H), 2.91–2.75 (m, 1H), 2.47 (dddd, J=18.0, 8.1, 6.1, 3.0Hz,...
Embodiment 3
[0062] A preparation method of 4-amino-2-(2-ethylamino) methyl butyrate dihydrochloride, comprising the steps:
[0063] (1) Preparation of 3-(2-bromoethyl)-dihydro-2(3H)-furanone
[0064] Add hydrobromic acid (2200g, 11.0mol, 10eq) into a 5-liter three-neck flask at room temperature, add tetrahydropyran-4-carboxylic acid (138.0g, 1.1mol, 1eq) under stirring, heat to 50°C for 12h, TLC After monitoring the reaction of raw materials, add 3000 milliliters of water to the reaction solution and extract three times with ethyl acetate (3 × 1000 mL), combine the organic layers, wash with water, wash with brine, dry over sodium sulfate, filter, spin the filtrate to obtain 194 g of yellow oil, It was directly put into the next step without purification, and the purity by HNMR was 95%.
[0065] 1 H NMR (400MHz, CDCl 3 )δ4.40 (td, J=8.9, 2.1Hz, 1H), 4.23 (ddd, J=10.3, 9.2, 6.4Hz, 1H), 3.72–3.62 (m, 1H), 3.49 (ddd, J=10.3, 8.0, 5.6Hz, 1H), 2.91–2.75(m, 1H), 2.47(dddd, J=18.0, 8.1, 6.1, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com