Patents
Literature
59 results about "Embolization Therapy" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Embolization therapy is a minimally invasive (non-surgical) treatment that occludes or blocks one or more blood vessels or vascular channels of malformations (abnormalities) such as:
Vagal stimulation for anti-embolic therapy
InactiveUS20060271115A1Increase volumeIncrease stimulationSpinal electrodesHeart defibrillatorsEmbolization TherapyBlood flow
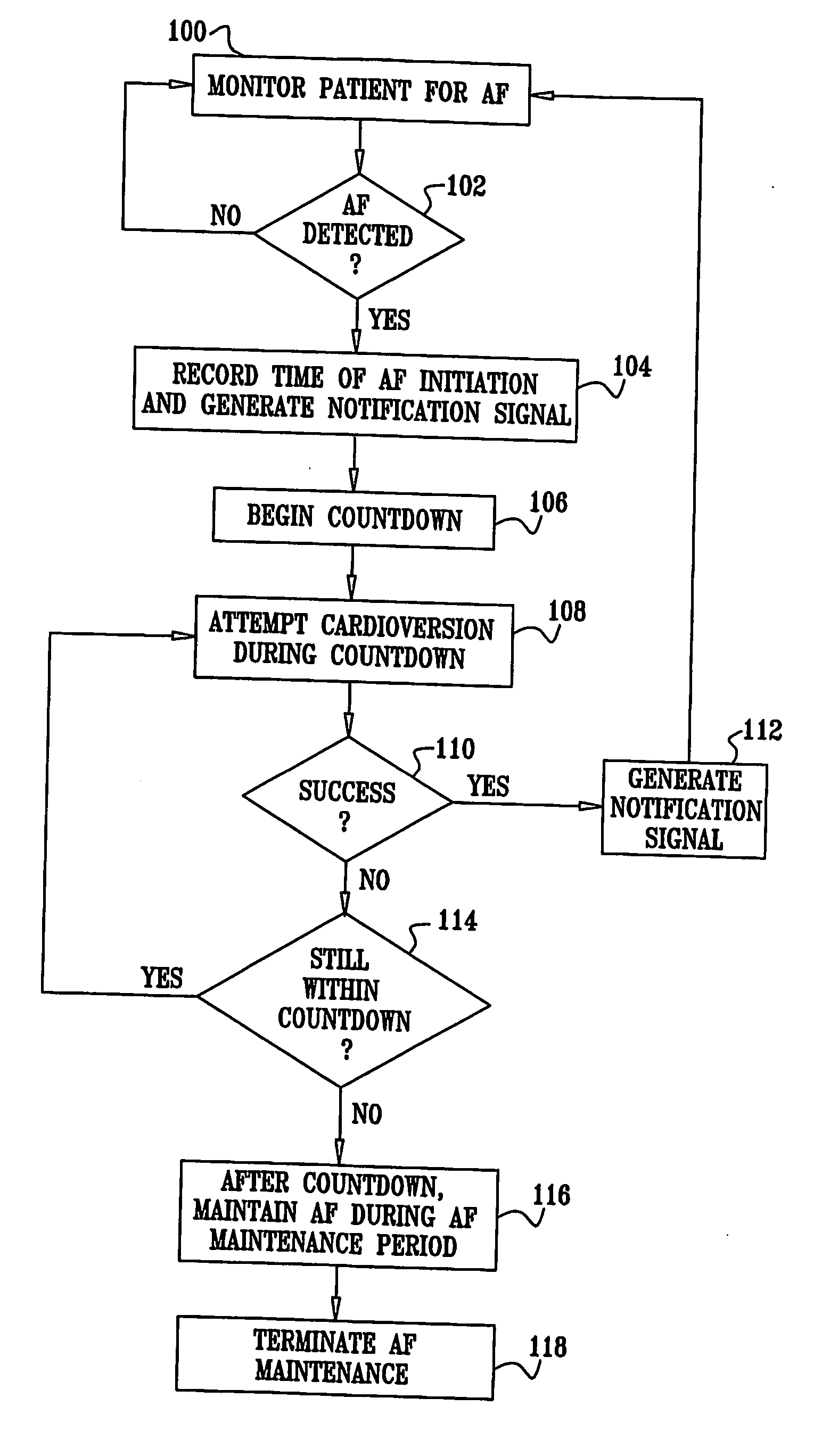
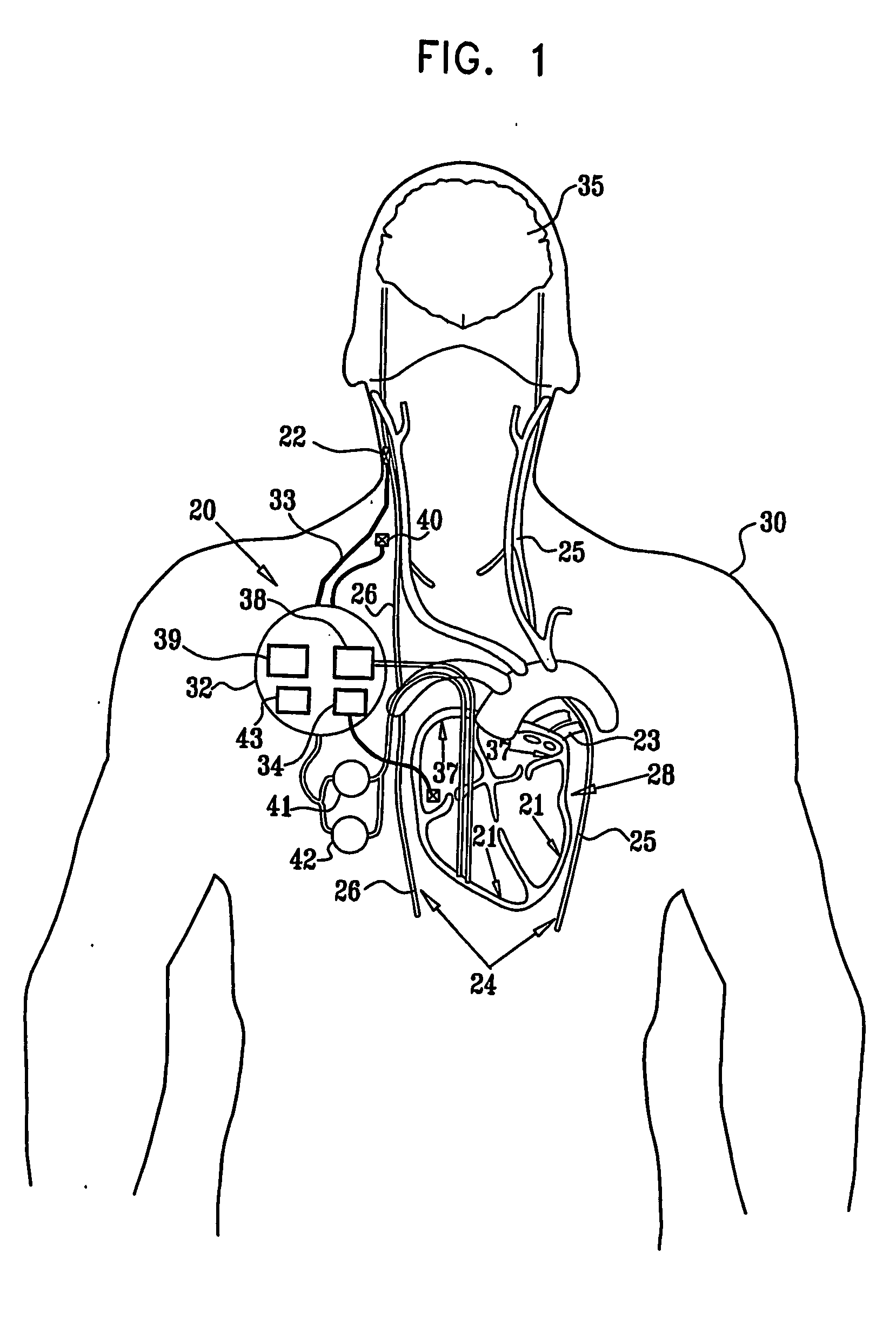
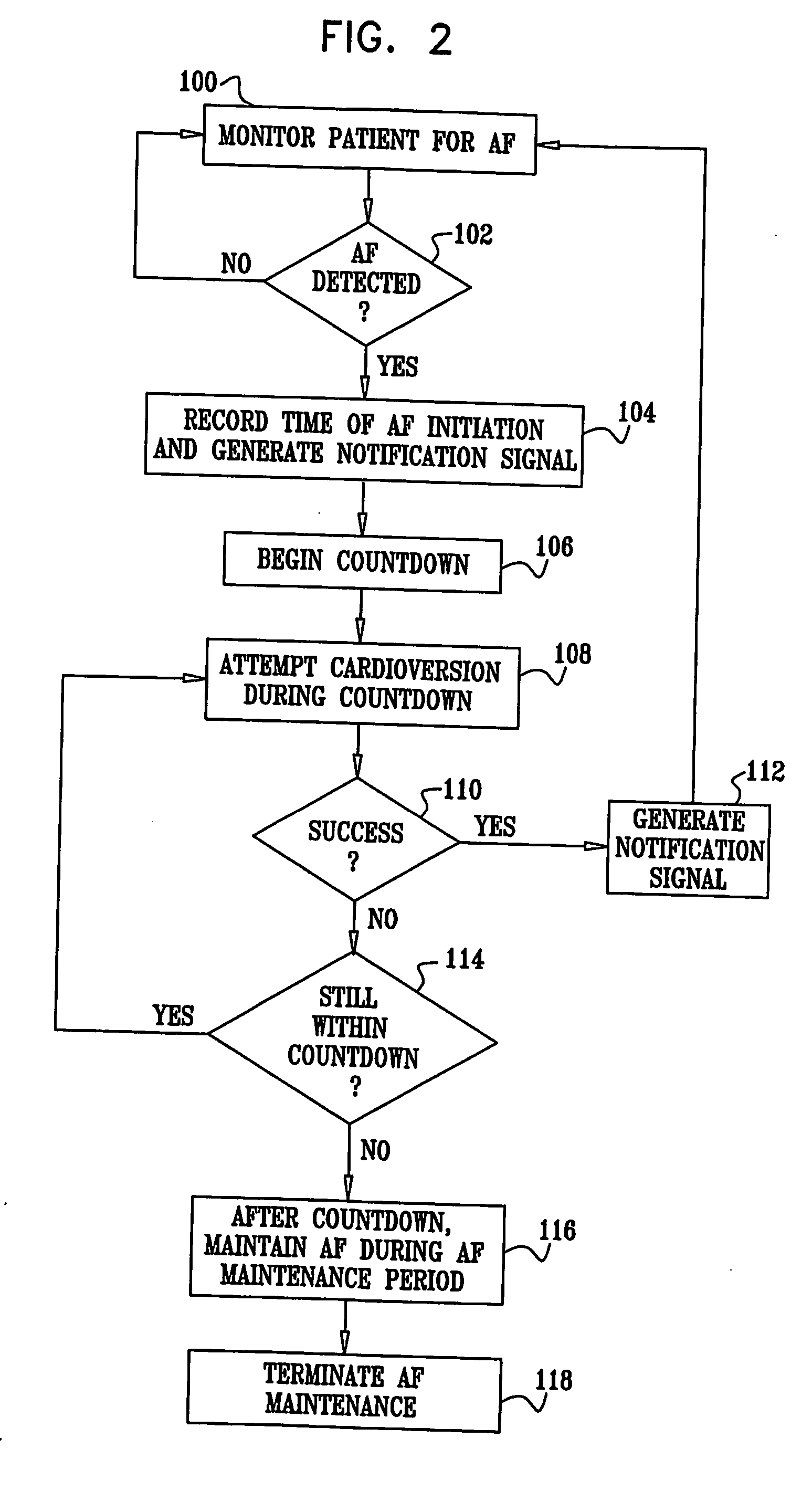
Apparatus (20) for treating a subject (30) suffering from spontaneous atrial fibrillation includes an electrode device (22), adapted to be coupled to a site of the subject (30) selected from the list consisting of: a vagus nerve (24) of the subject (30), an epicardial fat pad of the subject (30), a pulmonary vein of the subject (30), a carotid artery of the subject (30), a carotid sinus of the subject (30), a vena cava vein of the subject (30), and an internal jugular vein of the subject (30), and a control unit (32), adapted to drive the electrode device (22) to apply an electrical current to the site, and to configure the current to maintain the spontaneous AF for at least about 24 hours, so as to modify blood flow within the atria and reduce risk of thromboembolic events.
Owner:MEDTRONIC INC
Compositions and methods using microspheres and non-ionic contrast agents
ActiveUS20060251582A1Minimizing side-effectsImprove load effectAntibacterial agentsPowder deliveryAbnormal tissue growthEmbolization Therapy
The present invention relates to compositions and methods for treating diseases and disorders including cancer and various other angiogenic-dependent diseases, vascular malfunctions, arteriovenous malformations (AVM), hemorrhagic processes and treatment of pain, in particular tumor-related pain by drug delivery and / or therapeutic embolization using microspheres. More particularly the invention relates to microspheres containing non-ionic contrast agents, to compositions comprising these microspheres, as well as methods for preparing and using such compositions for embolization therapy. The invention further relates to compositions and methods using detectable microspheres for targeted drug delivery, irrespective of whether embolization is also needed.
Owner:BIOSPHERE MEDICAL SA (FR)
Detecting mutations for cancer screening and fetal analysis
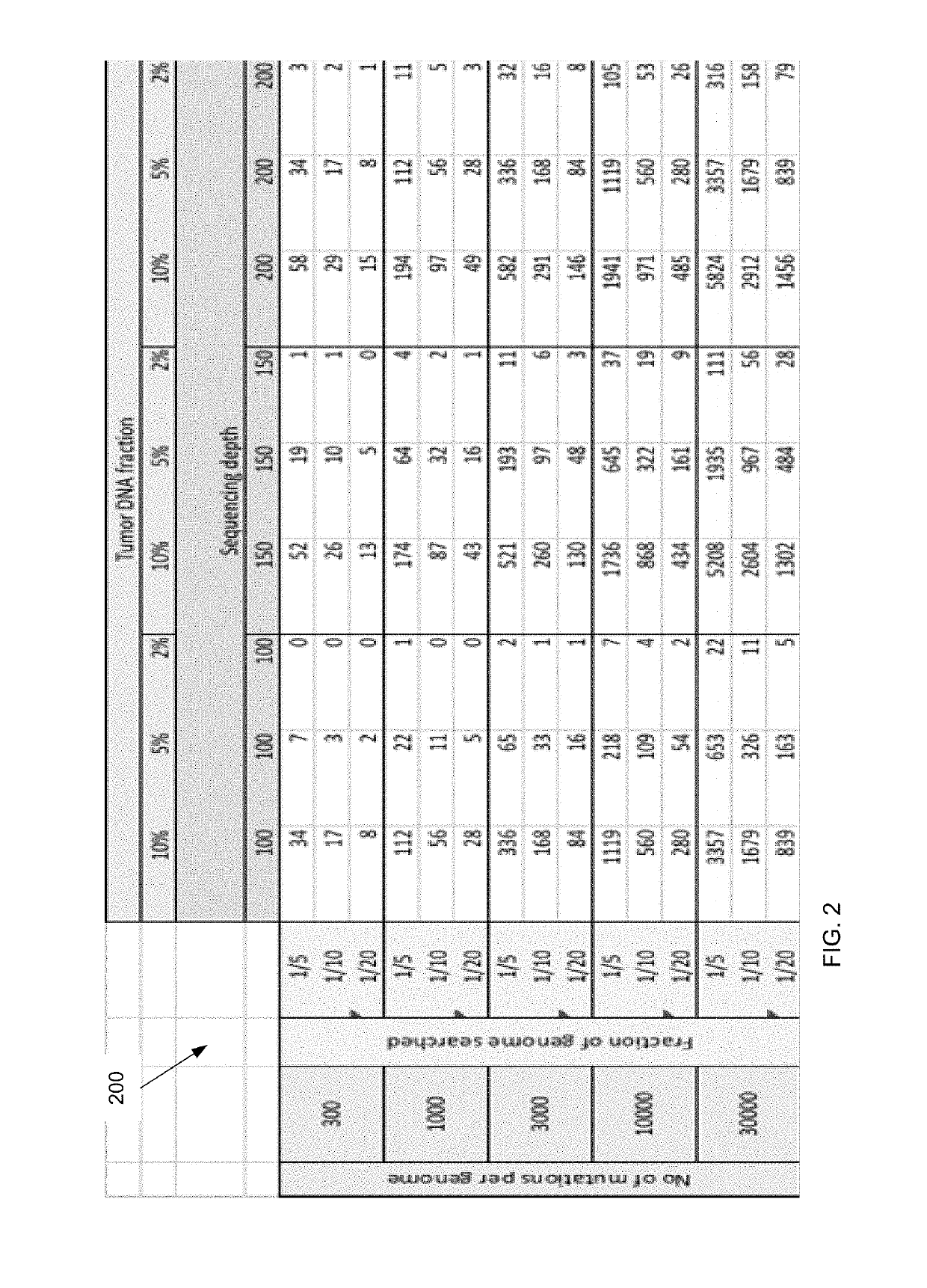
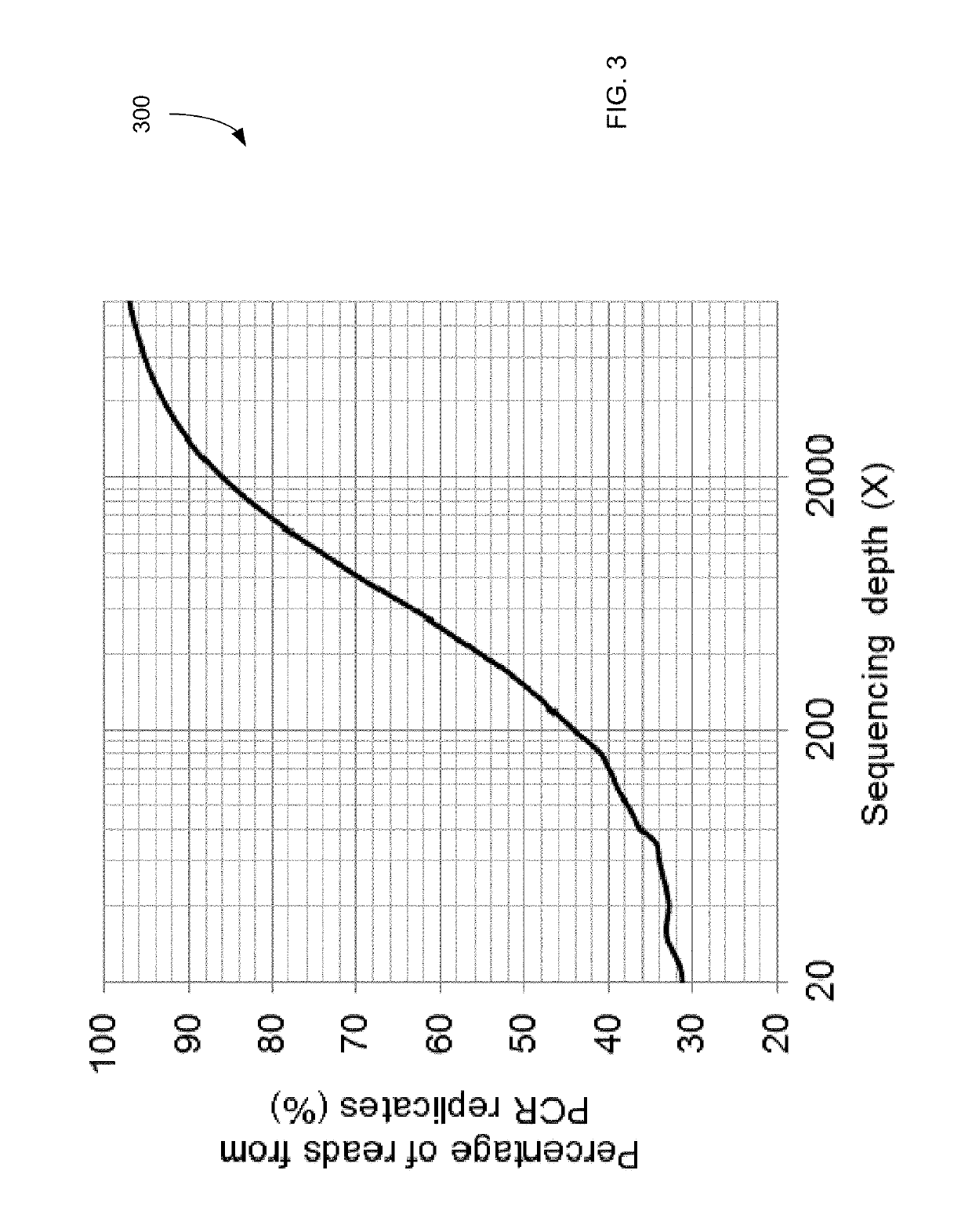
ActiveUS20170073774A1Accurate detectionMicrobiological testing/measurementProteomicsCell freeEmbolization Therapy
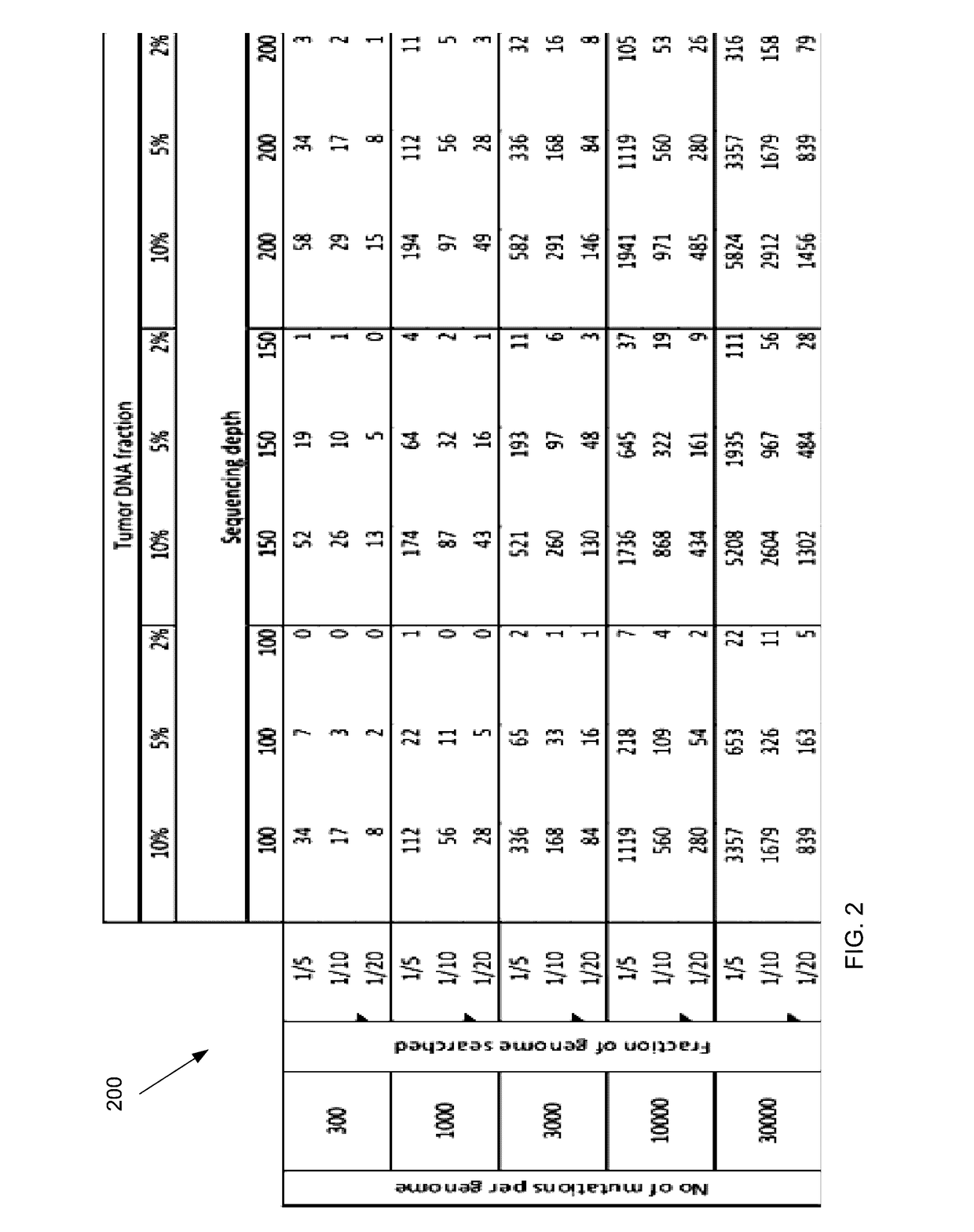
Embodiments are related to the accurate detection of somatic mutations in the plasma (or other samples containing cell-free DNA) of cancer patients and for subjects being screened for cancer. The detection of these molecular markers would be useful for the screening, detection, monitoring, management, and prognostication of cancer patients. For example, a mutational load can be determined from the identified somatic mutations, and the mutational load can be used to screen for any or various types of cancers, where no prior knowledge about a tumor or possible cancer of the subject may be required. Embodiments can be useful for guiding the use of therapies (e.g. targeted therapy, immunotherapy, genome editing, surgery, chemotherapy, embolization therapy, anti-angiogenesis therapy) for cancers. Embodiments are also directed to identifying de novo mutations in a fetus by analyzing a maternal sample having cell-free DNA from the fetus.
Owner:THE CHINESE UNIVERSITY OF HONG KONG
Quantitative perfusion analysis for embolotherapy
Methods for quantitative perfusion analysis for embolotherapy are presented. The method quantitatively measures blood flow changes based on angiographic information. The method may provide potential evaluation of optimal embolization endpoints in vascular vessels. The method may be used in various applications such as transcatheter arterial chemoembolization (TACE), or other medical procedures that affect blow flow within bodily tissues. The method is applicable towards treatment of tumors in liver, kidney, brain, and other organs.
Owner:UNITED STATES OF AMERICA +1
Compositions and methods using microspheres and non-ionic contrast agents
ActiveUS8226926B2Improve load effectMinimize the side-effects associatedAntibacterial agentsPowder deliveryDiseaseEmbolization Therapy
The present invention relates to compositions and methods for treating diseases and disorders including cancer and various other angiogenic-dependent diseases, vascular malfunctions, arteriovenous malformations (AVM), hemorrhagic processes and treatment of pain, in particular tumor-related pain by drug delivery and / or therapeutic embolization using microspheres. More particularly the invention relates to microspheres containing non-ionic contrast agents, to compositions comprising these microspheres, as well as methods for preparing and using such compositions for embolization therapy. The invention further relates to compositions and methods using detectable microspheres for targeted drug delivery, irrespective of whether embolization is also needed.
Owner:BIOSPHERE MEDICAL SA (FR)
Intracranial aneurysm interventional embolization treatment device
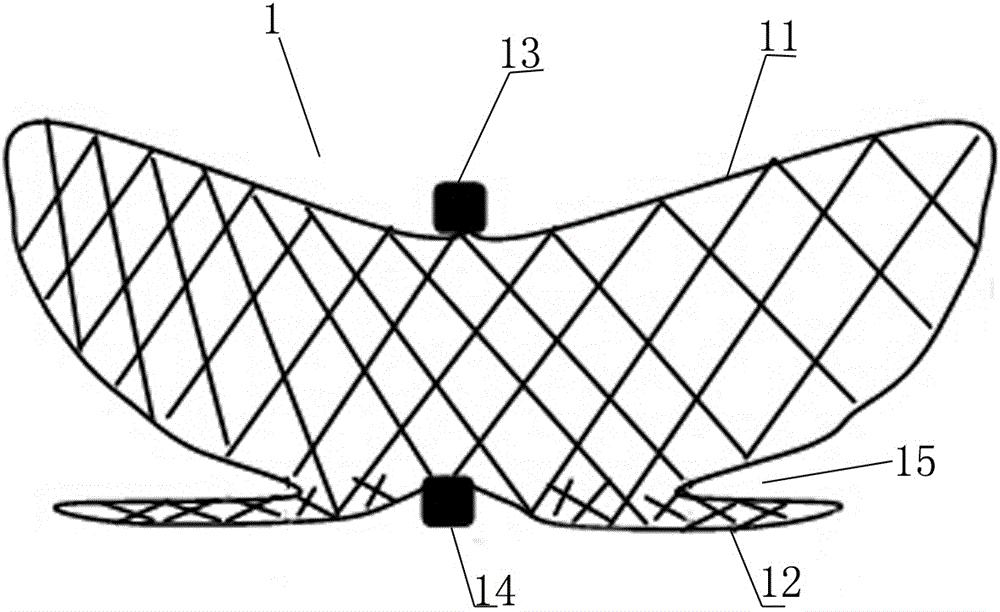
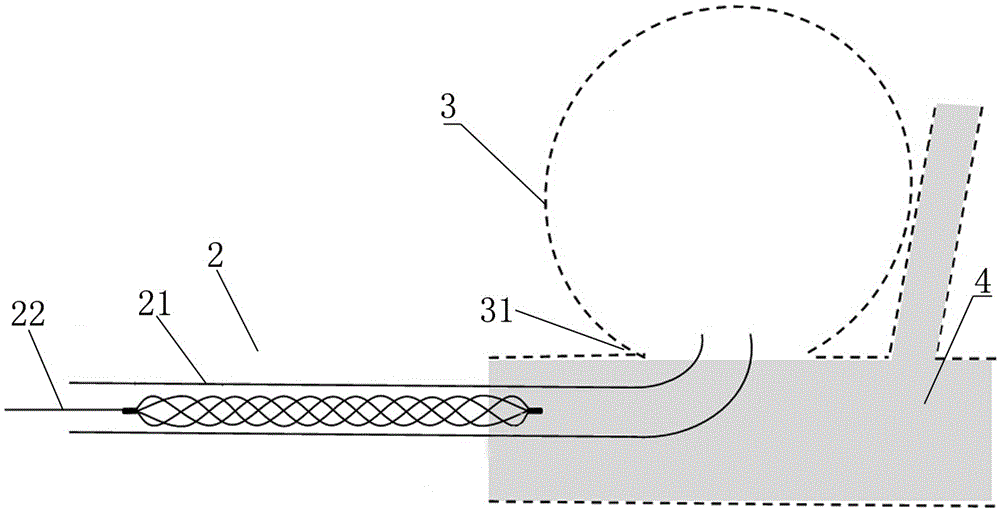
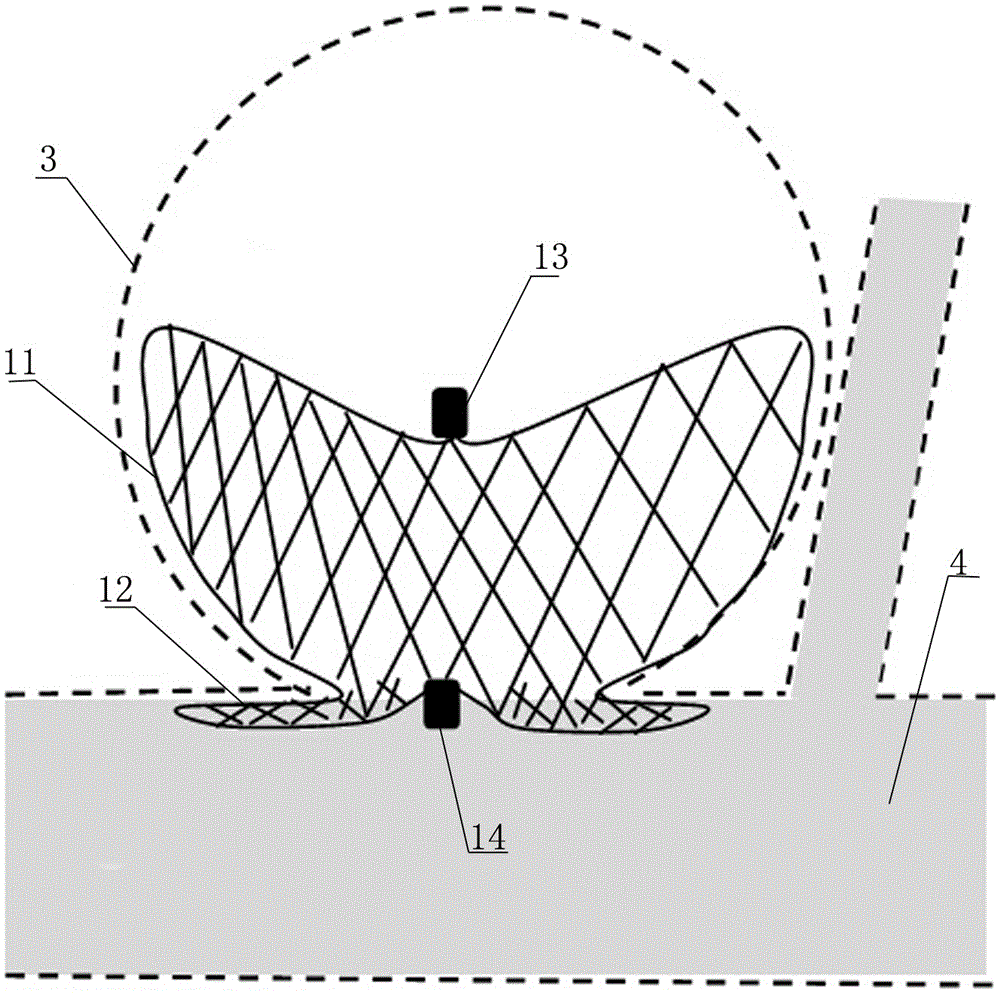
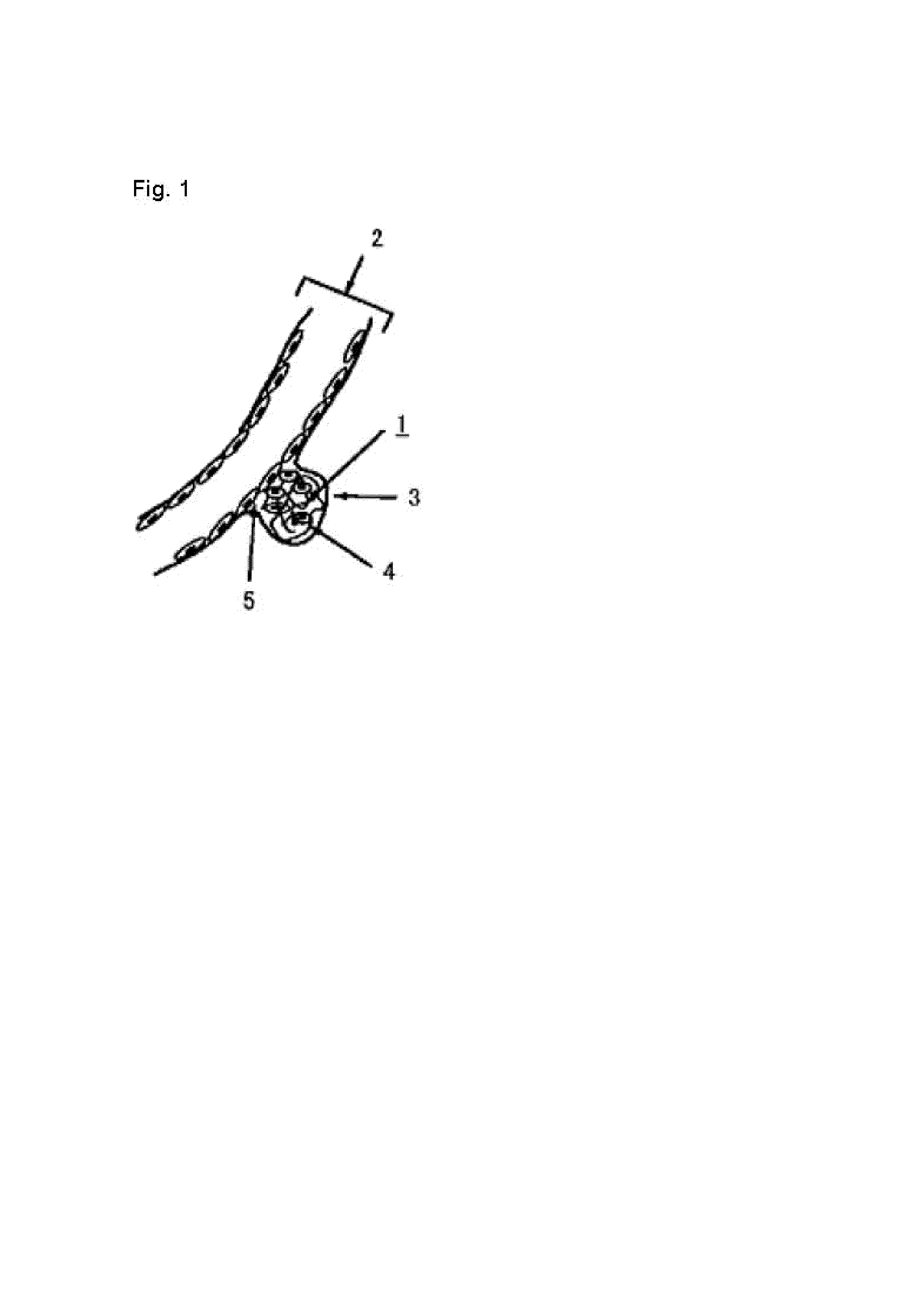
The invention discloses an intracranial aneurysm interventional embolization treatment device. The device comprises a plugging device and a conveying device. The plugging device is a net-shaped plugging object which is formed by knitting metal wires with a memory effect or high-elasticity polymer wires and developing wires. The plugging device comprises an upper part and a lower part which refer to an upper end intra-tumor part and a lower end extra-tumor part respectively. The upper end intra-tumor part and the lower end extra-tumor part are connected together to form a coating space for coating an aneurysmal neck. The conveying device comprises a conveying guide pipe and a conveying guide wire. In the conveying process, the plugging device is lengthened to be placed in the conveying guide pipe, and the conveying guide wire is connected with the tail of the plugging device. According to the intracranial aneurysm interventional embolization treatment device, intra-tumor thrombi can be induced to be formed, blood vessel repair of the aneurysmal neck is promoted, and therefore the purpose of healing arterial aneurysm is achieved. Moreover, parent blood vessels and normal branches cannot be affected obviously.
Owner:王奎重
Drug-loaded silica embolism microsphere and preparation method thereof
InactiveCN103751857AUniform and stable dispersionGood effectSurgeryPharmaceutical non-active ingredientsDiseaseHepatic tumor
Owner:TONGJI UNIV
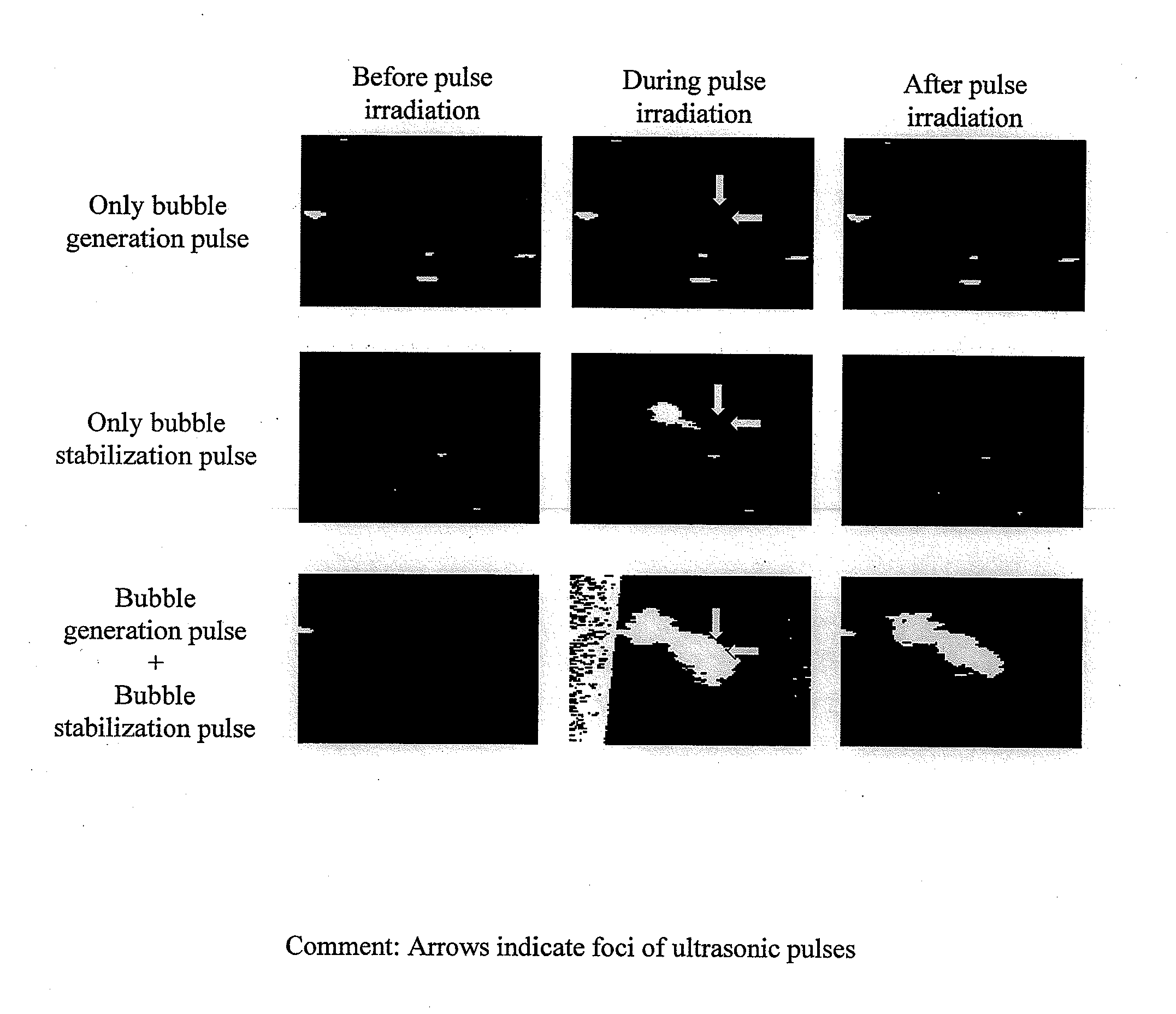
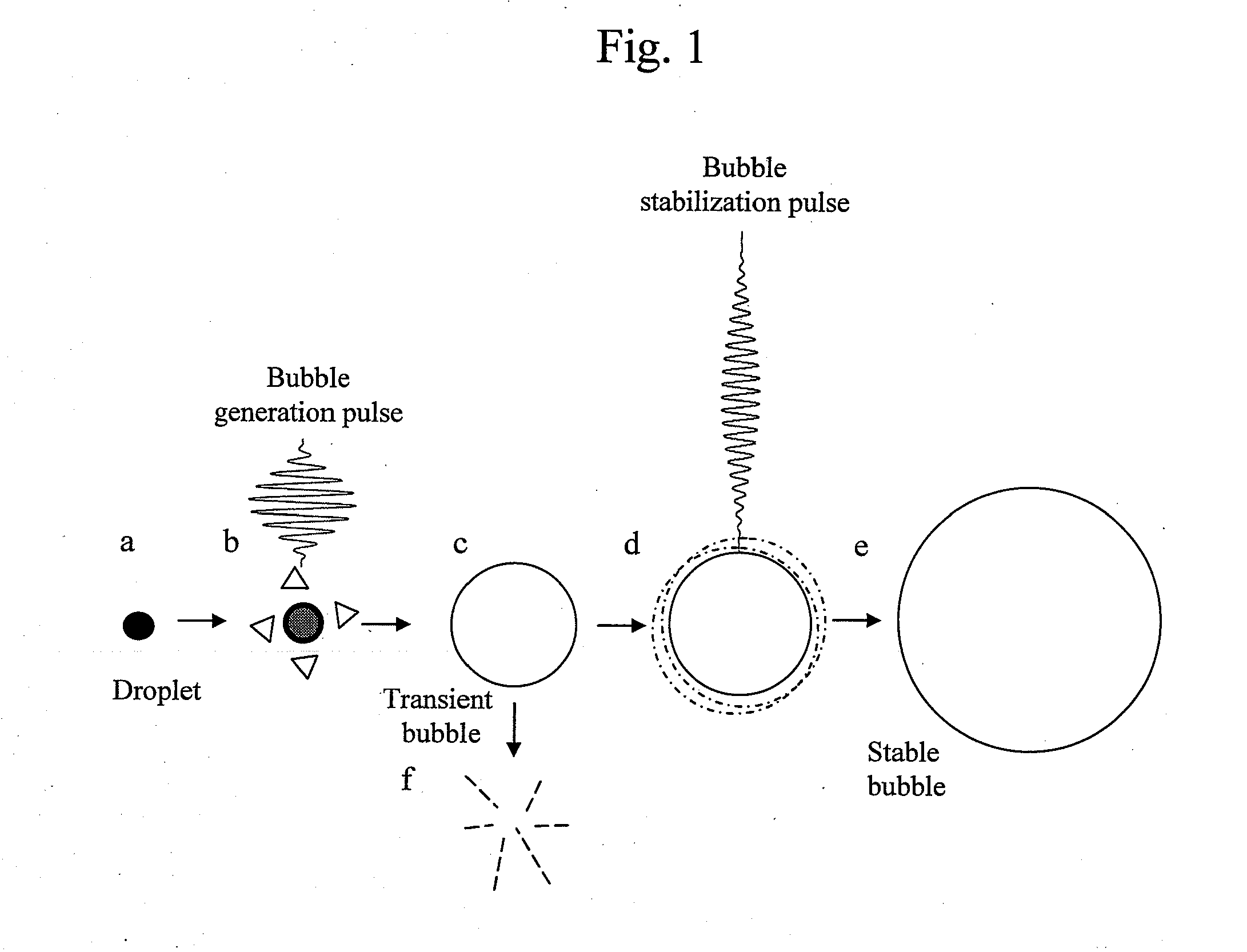
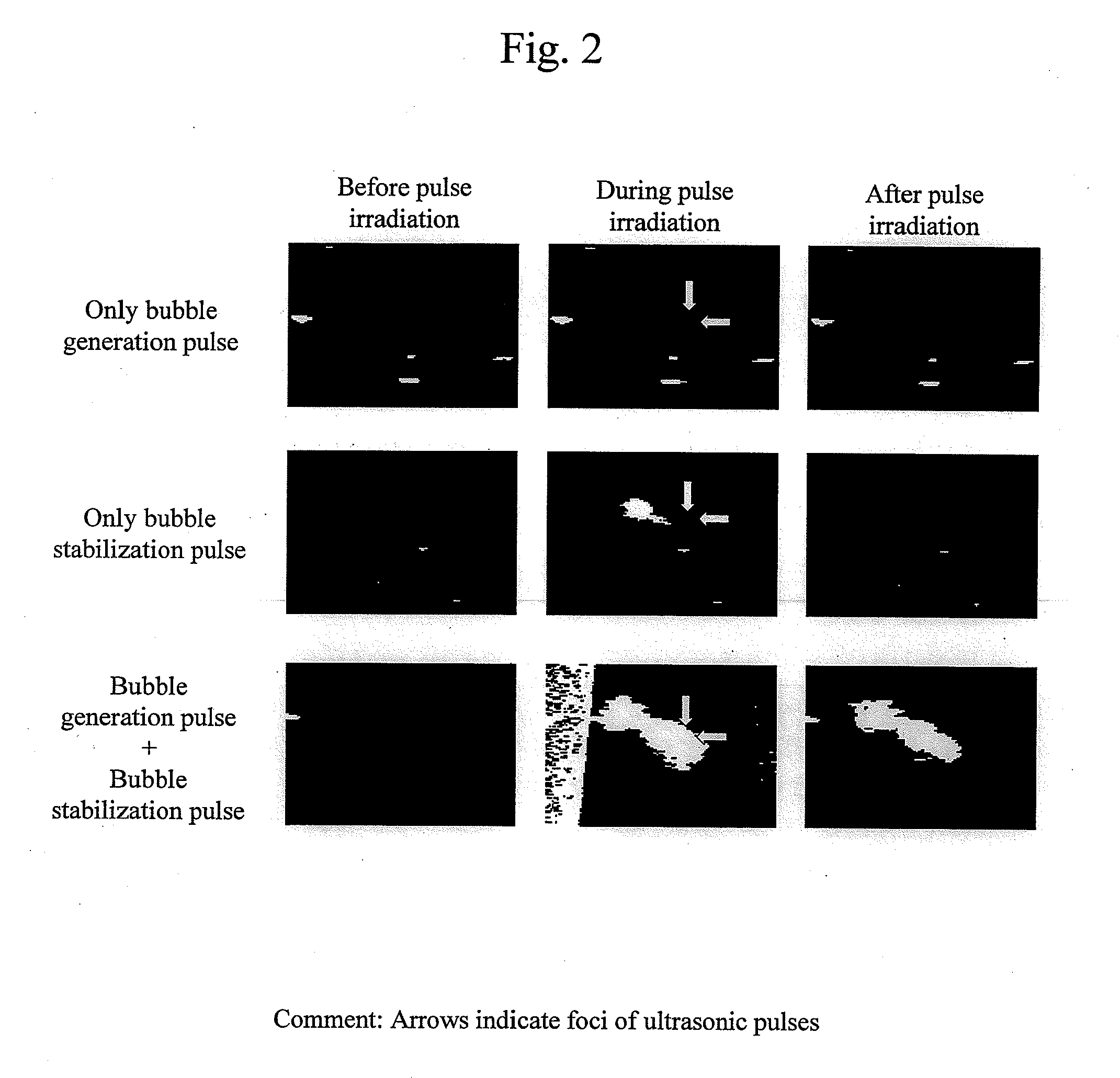
Ultrasonic treatment device
InactiveUS20120172720A1Minimally-invasiveHigh blood flow blocking effectUltrasonic/sonic/infrasonic diagnosticsUltrasound therapyEmbolization TherapyBlock effect
There is provided an ultrasonic treatment device that is minimally-invasive and that achieves embolization treatment having a high blood flow blocking effect. To this end, the ultrasonic treatment device is provided with a function to transmit to a target area of a subject both a bubble generation pulse that vaporizes a contrast agent, and a bubble generation pulse that causes the diameter of the formed bubble to increase.
Owner:HITACHI LTD
Magnetic resonance imaging detectable in-situ liquid crystal precursor embolism composition, preparation and application thereof
The invention discloses a magnetic resonance imaging detectable in-situ liquid crystal precursor embolism composition which comprises the following components: an MRI water-soluble or water-insoluble contrast medium, a liquid crystal material, a physiological acceptable water-soluble organic solvent and water. The following components can be added according to the treatment goal and the clinical demand: an X ray-impermeable water-soluble or water-insoluble contrast medium and / or drugs. The MRI detectable liquid embolism composition disclosed by the invention has the characteristics of low viscosity, no toxicity, low cost and fast curing, can be injected, and is mainly used in local embolism treatment and relevant imageological examination of tumors, vascular malformation and hemostasis.
Owner:HYGEA MEDICAL TECH CO LTD
Net basket type thrombus removing device
PendingCN112089477AQuick clearEffective and safe removalSurgeryPulmonary artery embolismCircular disc
The invention relates to the technical field of medical instruments, and discloses a net basket type thrombus removing device. The device comprises a far-end filtering disc, a near-end recycling discand at least one middle thrombus taking disc; the far-end filtering disc can be elastically expanded in the radial direction, and is provided with a net-shaped structure; the far-end filtering disc, the middle thrombus taking disc and the near-end recycling disc are sequentially and coaxially connected; in a natural unfolding state, the middle thrombus taking disc is in a flat disc shape; and thediameter of the middle thrombus taking disc is larger than that of the far-end filtering disc. According to the embodiment of the invention, a rapid, safe and effective thrombus removing mode can be provided for embolism treatment of pulmonary artery embolism and other large-size and thrombus-amount embolism, so that the treatment effect of acute middle-high risk pulmonary embolism patients can beimproved.
Owner:SHANGHAI TENDFO MEDICAL TECH CO LTD
Method for preparing degradable drug-loaded microsphere for embolization, and product obtained therefrom
ActiveUS20190192438A1Well siftedAccurate particle-size indicationOrganic active ingredientsSurgical adhesivesPolymer scienceOrganic solvent
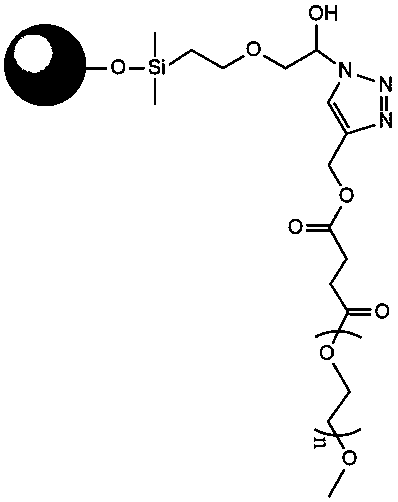
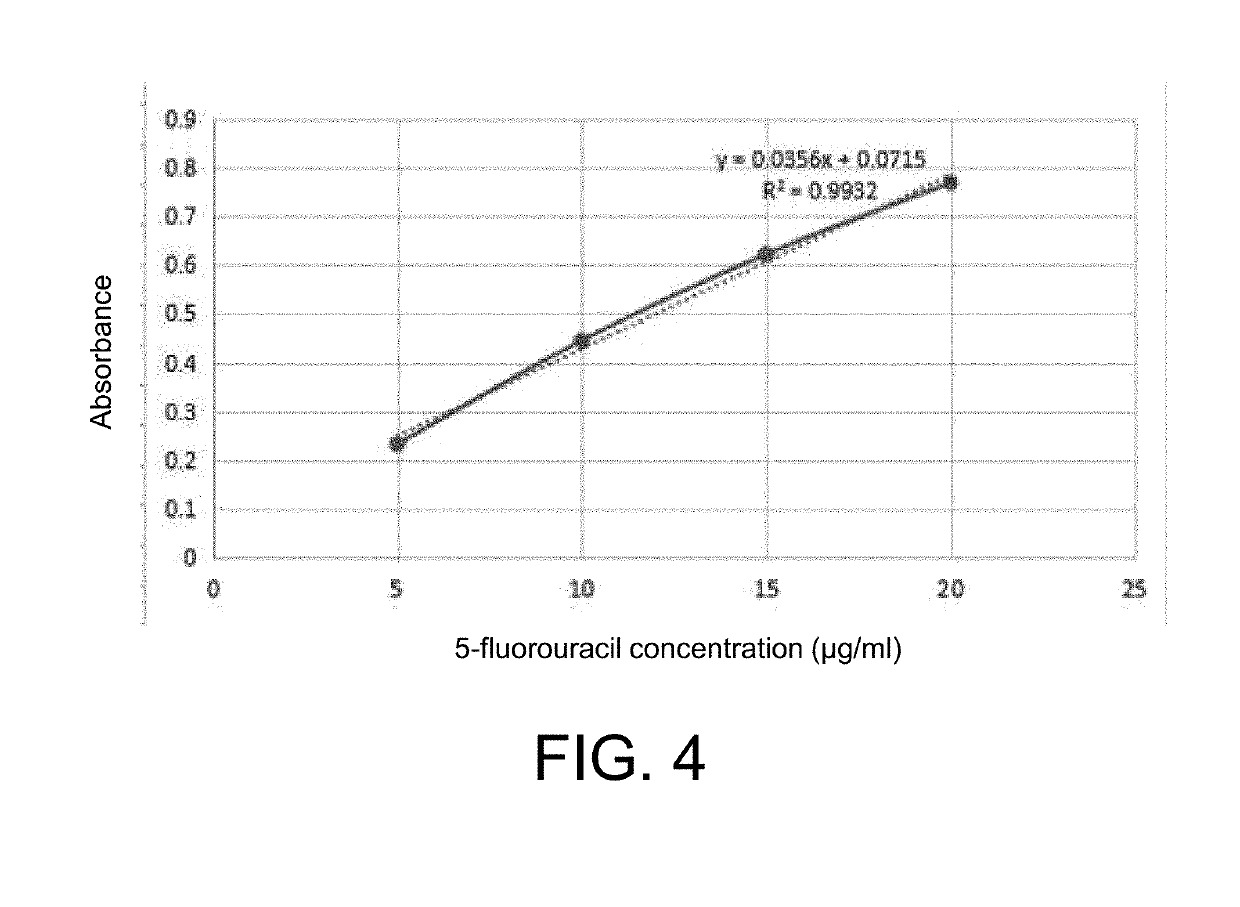
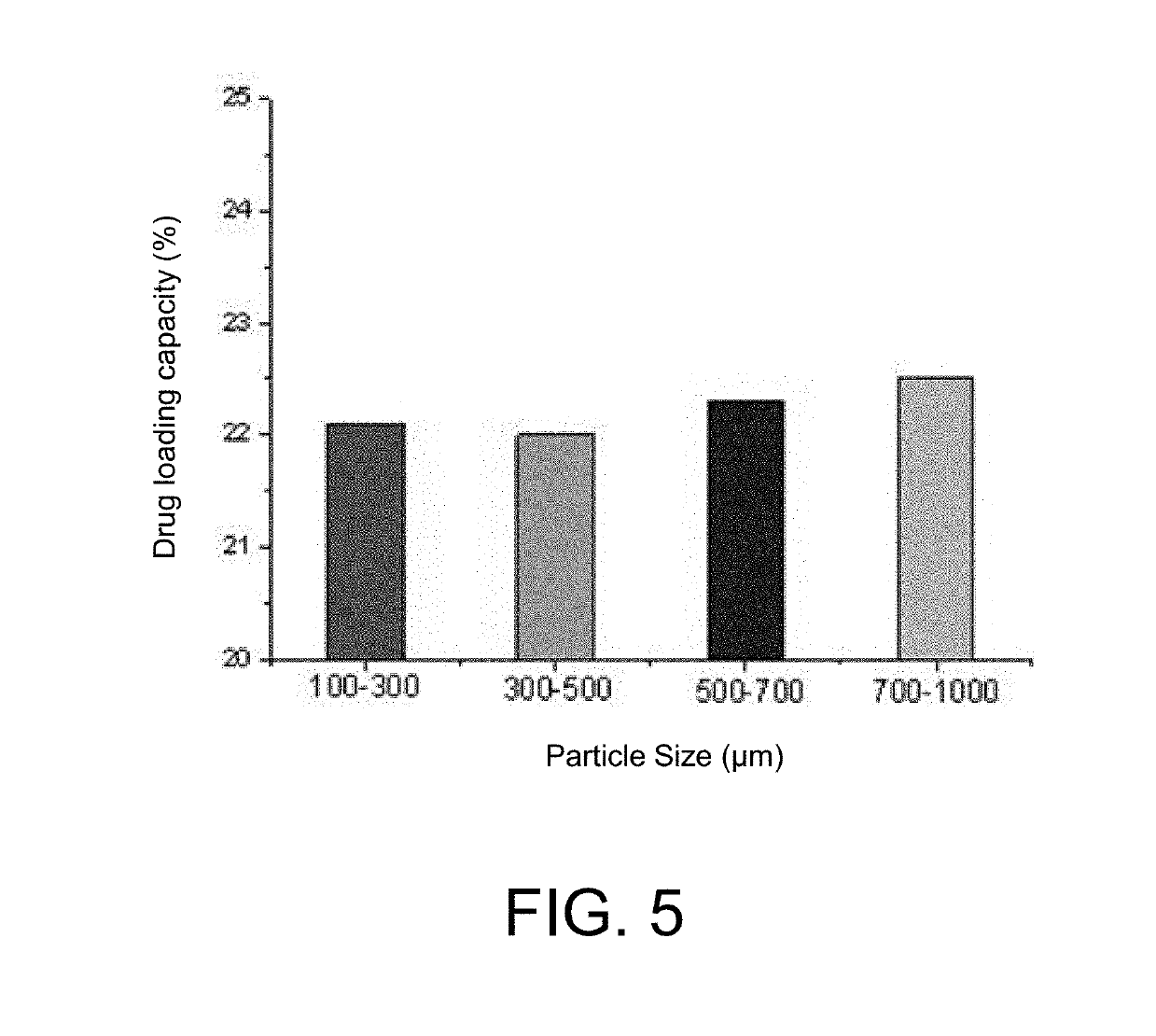
A method for preparing a degradable drug-loaded microsphere for embolization and a product obtained therefrom, includes the steps of: dissolving a degradable material in an organic solvent, then adding a drug and mixing well to form a suspension or solution; then pouring the drug-containing suspension or solution into an aqueous solution of polyvinyl alcohol, stirring, and thereafter adding water twice for dilution, to prepare the degradable drug-loaded microsphere. The microsphere prepared by the present invention has the advantages of having a controllable particle size, a high drug loading capacity, and a regular spherical shape, being convenient for sieve sizing and accurate particle-size indication, and being accurately targeted to an embolized blood vessel, and the like, and thus has a good application prospect in interventional embolization therapy.
Owner:SHANDONG RIENTECH MEDICAL TECH
Preparation method and use of liquid embolism agent having drug loading and developing functions
InactiveCN107899064AEasy to judge starting pointEasy to judge end pointSurgical adhesivesEmbolization TherapyEmbolization Agent
The invention discloses a preparation method and a use of a liquid embolism agent having drug loading and developing functions. The liquid embolism agent is formed through mixing anion-modified polyethylene-polyvinyl alcohol copolymer (EVOH), a solvent and a developer according to a certain ratio. Negative ion groups are introduced to the high molecular chain of the EVOH through a chemical modification or grafting modification technology, and the negative ion groups can adsorb and load cationized chemotherapeutic anticancer drugs through positive and negative charge interactions. The solvent can be dimethyl sulfoxide (DMSO), N-methylpyrrolidone (NMP) or ethanol or a mixture of the DMSO, NMP and ethanol. The developer is a metallic tantalum micro-powder or an iodine-containing contrast agent or a mixture of the metallic tantalum micro-powder and the iodine-containing contrast agent. The liquid embolism agent can be widely applied to interventional embolization therapy, and is especiallysuitable for transcatheter arterial chemoembolization (TACE) therapy of liver cancer.
Owner:杭州华微医疗科技有限公司
Detecting mutations for cancer screening
Embodiments are related to the accurate detection of somatic mutations in the plasma (or other samples containing cell-free DNA) of cancer patients and for subjects being screened for cancer. The detection of these molecular markers would be useful for the screening, detection, monitoring, management, and prognostication of cancer patients. For example, a mutational load can be determined from the identified somatic mutations, and the mutational load can be used to screen for any or various types of cancers, where no prior knowledge about a tumor or possible cancer of the subject may be required. Embodiments can be useful for guiding the use of therapies (e.g. targeted therapy, immunotherapy, genome editing, surgery, chemotherapy, embolization therapy, anti-angiogenesis therapy) for cancers. Embodiments are also directed to identifying de novo mutations in a fetus by analyzing a maternal sample having cell-free DNA from the fetus.
Owner:THE CHINESE UNIVERSITY OF HONG KONG
Sodium alginate targeted sustained release microsphere vascular occlusive agent containing Sorafenib as well as preparation and application thereof
ActiveCN101536986ALow cost of treatmentStrong targetingSurgeryPharmaceutical product form changeSide effectEmbolization Agent
The invention discloses a sodium alginate targeted sustained release microsphere vascular occlusive agent containing Sorafenib as well as a preparation and application thereof. The invention is characterized by comprising carrier sodium alginate and antitumor targeted drug Sorafenib; the sodium alginate wraps the Sorafenib; the Sorafenib is 1 portion by weight and the sodium alginate is 1 to 30 portions by weight. The vascular occlusive agent leads the oral targeted drug to achieve the purpose of interventional embolotherapy by an interventional embolotherapy method for changing preparation formulation and changing the drug administration way, simultaneously leads Sorafenib of the antitumor drug to be directly located to cancer tissues in a fast and long-acting manner from local target areas, has strong pointedness and obvious effect, does not damage normal tissues while cutting blood supply of tumor and inhibiting growth of the tumor cells, has high drug concentration in local target areas and little drug amount for the whole body, better and effectively protects the self immune system of patients and reduces toxic and side effects and the like.
Owner:BEIJING SHENGYIYAO SCI & TECH DEV
Drug sustained-release alginic acid embolism microsphere and preparation method thereof
InactiveCN111773428AEvenly distributedAchieve in situ controlled releaseSurgical adhesivesEmbolization TherapyMicrosphere
The invention discloses a drug sustained-release alginic acid embolism microsphere and a one-step electrostatic spraying preparation method thereof. Sodium alginate is compounded with a chemotherapeutic drug with positive charges, and the drug-loaded alginic acid embolic microsphere is prepared through a multi-nozzle one-step electrostatic spraying technology, so that combined treatment of embolism treatment and chemotherapy is realized. According to the multi-nozzle one-step electrostatic spraying preparation method provided by the invention, the drug sustained-release embolism microsphere can be prepared in one step, the prepared microsphere is uniform in particle size and controllable in size, the diameter is 100-1000 microns, and the microsphere has the potential of large-scale production. According to the alginic acid embolism microsphere provided by the invention, the drug loading capacity of common embolism microspheres is greatly improved, long-term slow release of chemotherapeutic drugs can be realized, nutrient source blood supply required by tumor growth can be blocked, the local drug concentration of a focus part can be improved, and meanwhile, the drug concentration inother organs is reduced, toxic and side effects are reduced, synergistic treatment of embolism treatment and chemotherapy is achieved, and the method has great development prospects.
Owner:HUAZHONG UNIV OF SCI & TECH
Intracranial dense net stent
InactiveCN112971903AEffective blockingEasy to operateOcculdersAntithrombotic treatmentEmbolization Therapy
The invention discloses an intracranial dense mesh stent with an anticoagulant coating. The intracranial dense mesh stent comprises a dense mesh stent body and a hydrophilic polymer material covering the dense mesh stent body, wherein the dense mesh stent body is coated with the anticoagulant coating. According to the intracranial dense net stent, not only can a channel be reconstructed for blood vessels, but also antithrombotic treatment can be carried out on the blood vessel wall at the position of stent implantation, and embolism treatment can be carried out on aneurysm at the same time.
Owner:XINKAINUO MEDICAL TECH SHANGHAI CO LTD
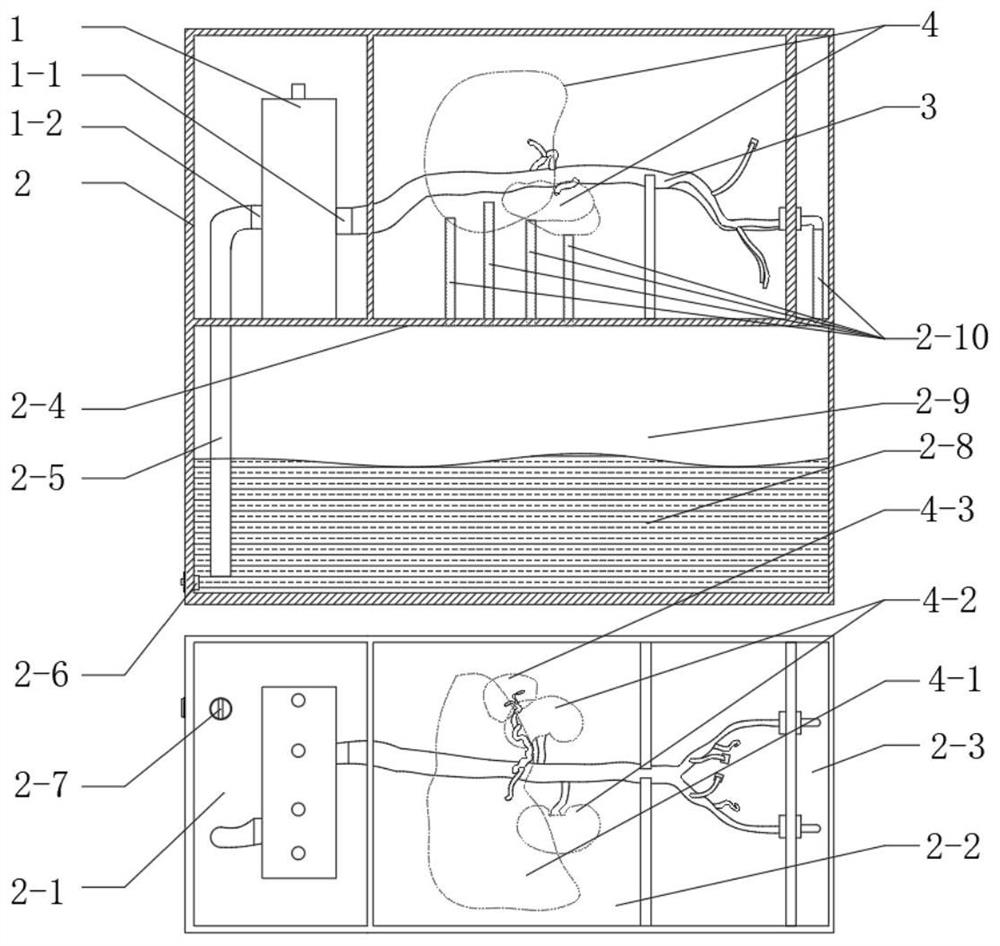
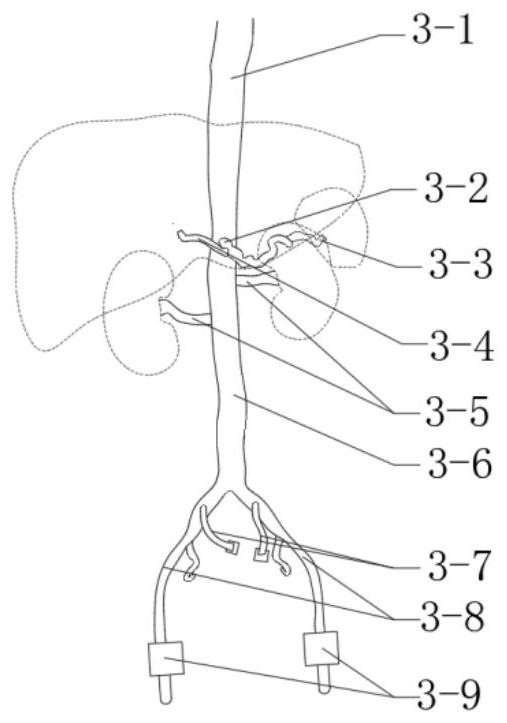
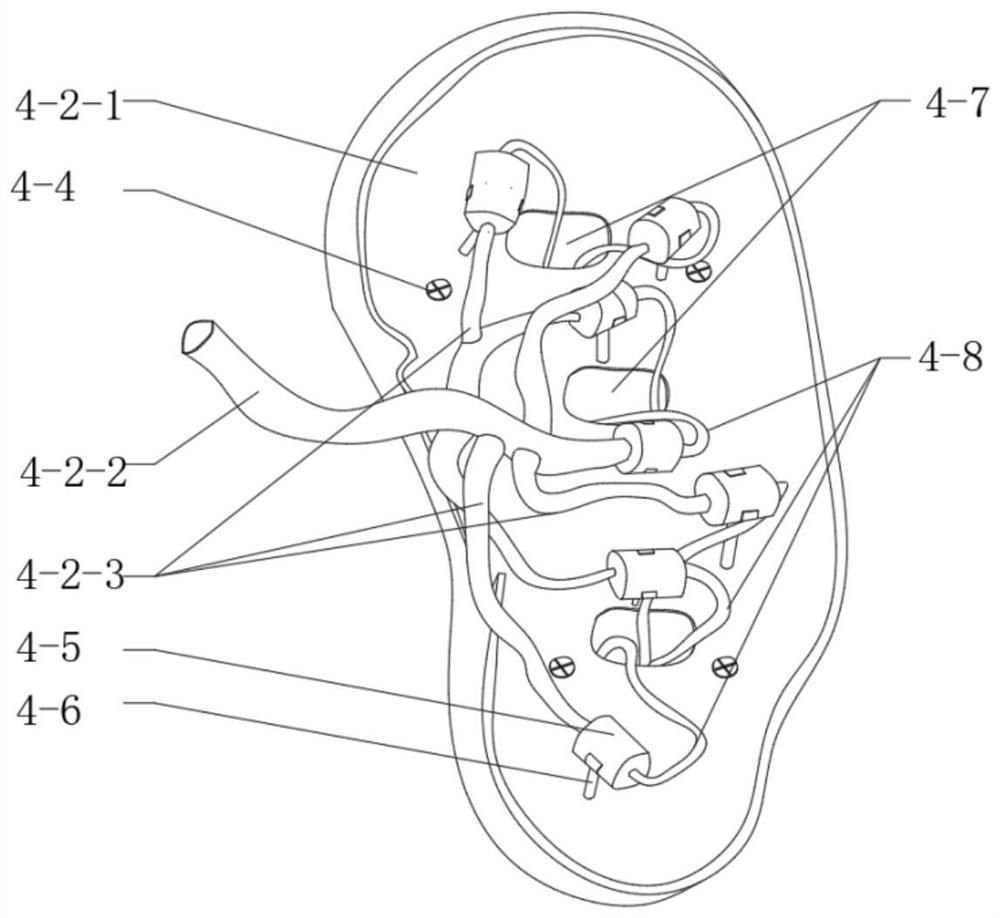
In-vitro simulation device for vascular intervention embolism treatment
PendingCN112562474AHigh degree of reductionSimulation is accurateEducational modelsEmbolization TherapyKidney arteries
The invention belongs to the technical field of medical test instruments and particularly relates to an in-vitro simulation device for vascular intervention embolism treatment. The device comprises apulse pump, an organ artery module, a basic blood vessel module and a supporting structure, wherein the pulsation pump is used for supplying blood to the simulated heart pulsation; the organ artery module comprises a renal artery module, a hepatic artery module and a splenic artery module which are respectively used for simulating renal artery embolism, hepatic artery embolism and splenic artery embolism; the basic blood vessel module is used for simulating a basic blood vessel passage for the interventional catheter to enter the body; and the supporting structure is used for supporting and fixing the modules and maintaining the shape of blood vessels. The device is high in blood vessel reduction degree, can be modified according to the specific illness state, is transparent and visual, and can accurately simulate the embolism part and the embolism process.
Owner:FUDAN UNIV
Multifunctional liquid metal embolizing agent as well as preparation and application thereof
ActiveCN111514368AFast preparationEasy to prepareSurgical adhesivesEnergy modified materialsMicrosphereEmbolization Agent
The invention provides a multifunctional liquid metal embolizing agent as well as a preparation and an application thereof. The multifunctional liquid metal embolizing agent is prepared from liquid metal, functional nanoparticles and a high polymer material, wherein the liquid metal is spherical and forms an inner core of the embolization agent; the functional nanoparticles are dispersed on the surface of the liquid metal; and the high polymer material coates outside the inner core and the functional nanoparticles. The multifunctional liquid metal embolization agent provided by the invention can realize the functions of embolization, CT imaging, MRI imaging, drug loading, thermal conversion, magnetism and the like, and is expected to realize the combination of targeted embolization treatment with chemotherapy, thermal therapy and radiotherapy; and the preparation method of the multifunctional liquid metal microsphere embolization agent provides possibility for more quickly and conveniently preparing the multifunctional microsphere embolization agent with multiple curative effects and diagnosis and treatment integration.
Owner:TECHNICAL INST OF PHYSICS & CHEMISTRY - CHINESE ACAD OF SCI
Statin-loaded coils for acceleration of organization after endovascular coiling of aneurysms
InactiveUS20130178892A1Improve securityEasy to produceDilatorsTissue regenerationEmbolization TherapyNiobium
The present invention provides a vascular treatment material which has high safety, and also can exert high organization acceleration effect. The present invention also provides a commercially available vascular treatment material, which is easy to produce and can be easily sterilized, and also can be stored for a long period. Disclosed is a vascular treatment material including a coil on which statin is loaded. It is preferred that a wire forming the coil is made of at least one kind of metal selected from platinum, tungsten, gold, cobalt, chromium, titanium, niobium, aluminum, tantalum, iron, nickel and the like. The vascular treatment material of the present invention is particularly useful, which is placed in a blood vessel for embolization treatment, or which accelerates organization for prevention of recanalization of aneurysm or for prevention of rupture of aneurysm.
Owner:KYOTO UNIV
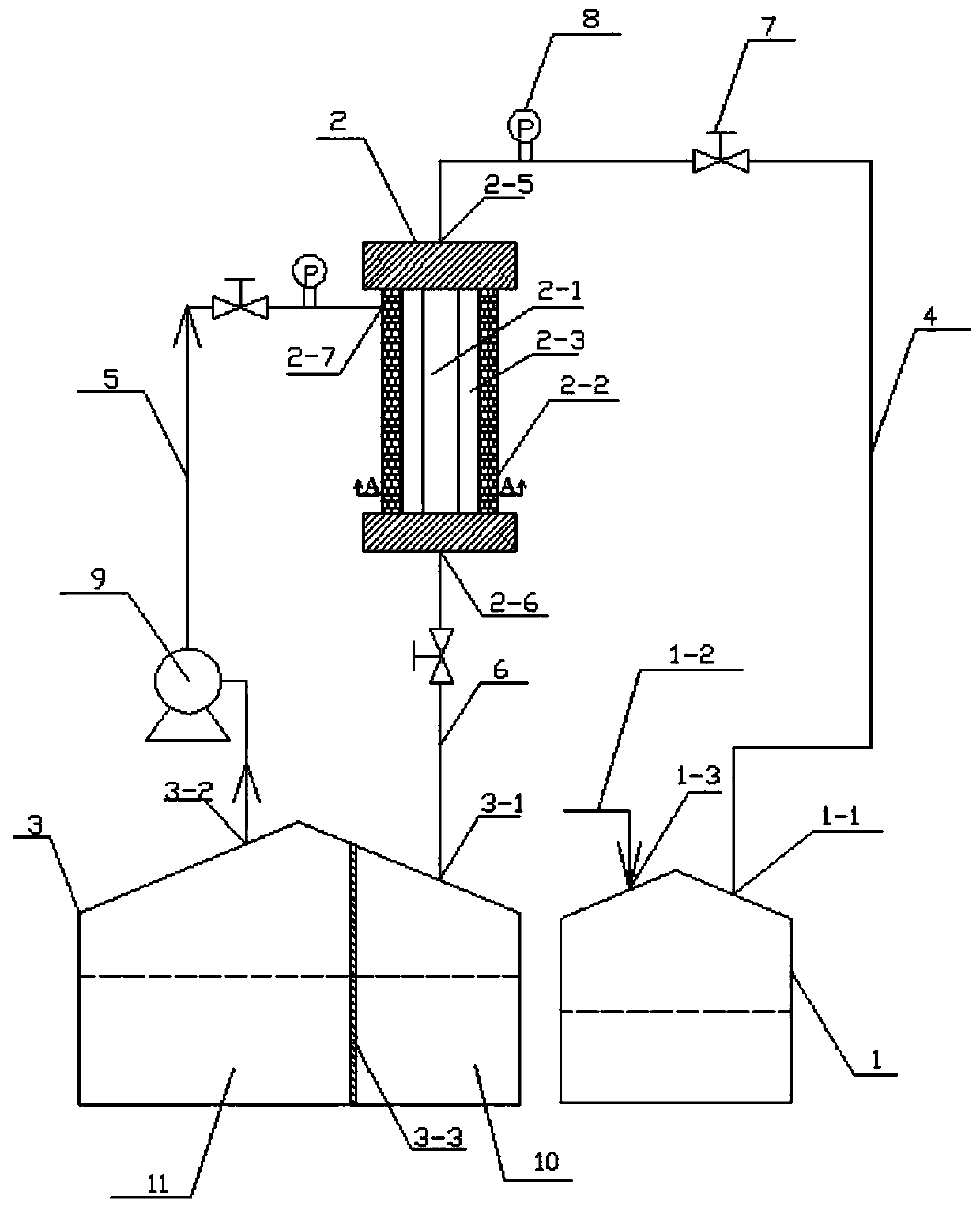
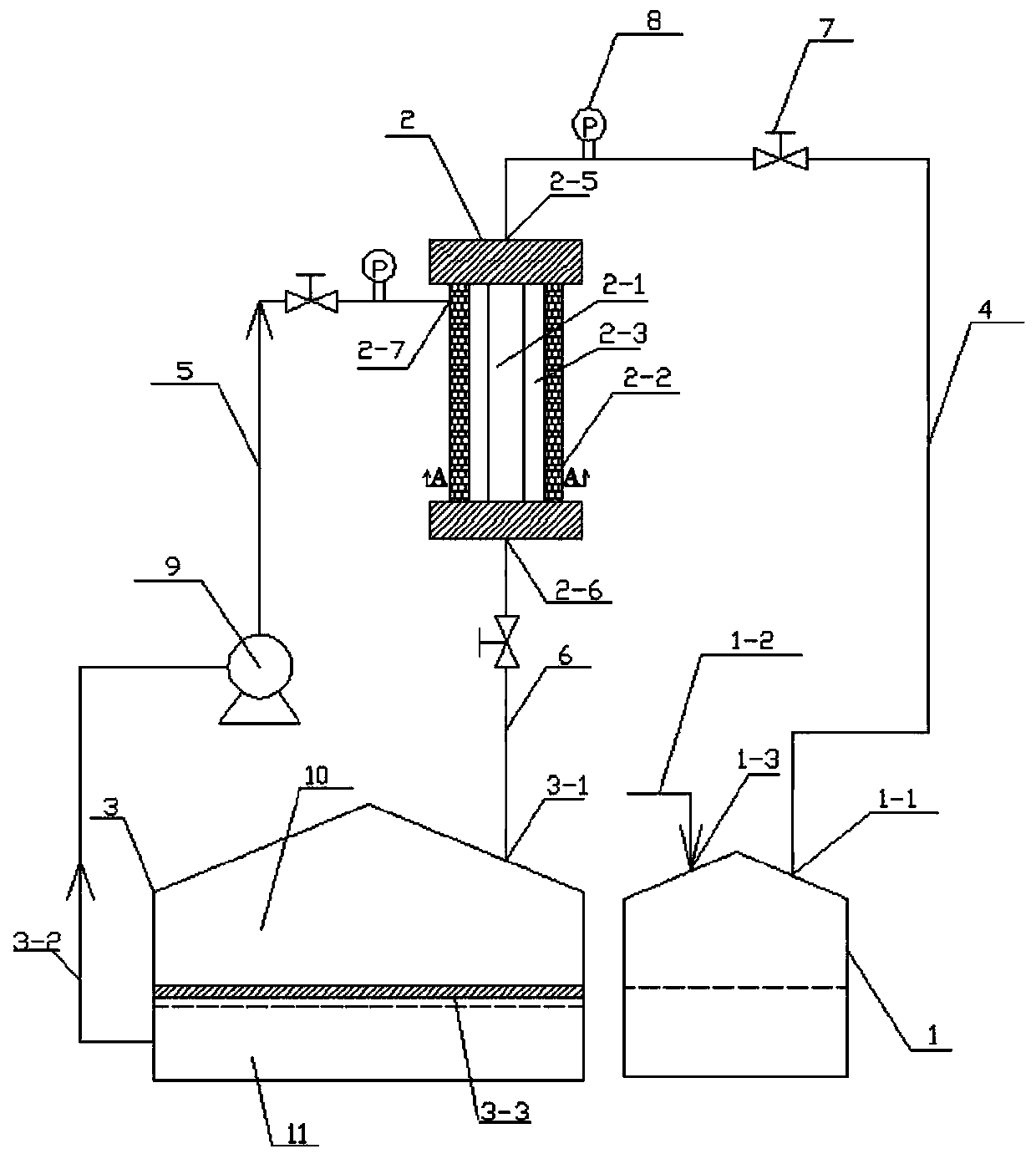
Method and device for preparing degradable microspheres for embolotherapy
ActiveCN111298187ALower glass transition temperatureFast hardeningSurgical adhesivesMicroballoon preparationStationary phaseMicrosphere
The invention belongs to the technical field of medicine preparation, and particularly relates to a method and a device for preparing degradable microspheres for embolotherapy. The method comprises the steps of dissolving PCL (polycaprolactone) or PHA (polyhydroxyalkanoate) in an organic solvent to form a stationary phase; and extruding the stationary phase into a mobile phase which flows continuously and circularly through micropores under pressure, enabling the extruded stationary phase to be separated from the micropores to form the microspheres insoluble in the mobile phase under the impulsive force effect of the mobile phase on an interface in contact with the mobile phase, collecting the microspheres, and recycling the mobile phase. Alcohol is adopted as the mobile phase, the hardening speed on the surfaces of the microspheres is increased, a water phase is not used, only a small amount of the mobile phase is used, sewage treatment is not needed, a corresponding device is supplemented, the mobile phase is recycled and finally recyclable, the amount of the mobile phase used for preparing equivalent microspheres is reduced, and the method is easy for industrial production.
Owner:山东采采医疗科技有限公司
MRI development embolism microballoon based on gadolinium alginate
InactiveCN103861157AGood biocompatibilityPromote degradationSurgeryEmulsion deliveryMicrosphereLanthanide
The invention discloses preparation of a magnetic resonance imaging (MRI) developpable microbead and application potential of the microbead to tumour arterial embolism treatment. Sodium alginate is taken as a raw material, the microbead is formed by employing an electrostatic drop generator, and on the basis, lanthanide metal ion such as Gd<3+> is introduced as a development group for MRI imaging, so that visualization of embolism treatment process is realized.
Owner:DALIAN INST OF CHEM PHYSICS CHINESE ACAD OF SCI
Super-smooth nickel-titanium alloy intracranial vascular stent with micro-nano structure
ActiveCN112535560ADifferent mechanical propertiesImprove flexibilityStentsProsthesisEmbolization TherapyIntracranial vascular stent
The invention relates to a super-smooth nickel-titanium alloy intracranial vascular stent with a micro-nano structure, can be used for assisting a spring ring embolism in treating intracranial aneurysm, and belongs to the field of medical instruments. An intracranial vascular stent body is composed of an open-loop structure and a closed-loop structure; the open-loop structure is formed by connecting first V-shaped units distributed in the circumferential direction and second V-shaped units distributed in the circumferential direction through connecting rods; the closed-loop structure is formedby connecting second V-shaped units distributed in the circumferential direction through connecting rods; the number ratio of the open-loop free ends of the second V-shaped units to the open-loop free ends of the first V-shaped units is 1 to 2; and the open-loop structure enhances the flexibility and adherence of the stent, and the closed-loop structure provides supporting performance. The intracranial vascular stent has super-smooth performance, can be suitable for assisting spring ring embolism in treating aneurysms (such as large-curvature blood vessels and variable-diameter blood vessels)at intracranial complex blood vessels, and achieves a good adherence effect.
Owner:INST OF METAL RESEARCH - CHINESE ACAD OF SCI
High-drug-loading degradable alginate sulfate vascular embolization microspheres and preparation method and application thereof
PendingCN113730646AGood biocompatibilityPromote degradationSurgical adhesivesEmbolization TherapyEmbolization Agent
The invention discloses a preparation method of high-drug-loading degradable alginate sulfate vascular embolization microspheres. The preparation method comprises the following steps of: S01, preparing an aqueous phase; S02, preparing an oil phase; S03, preparing alginate sulfate microspheres; and S04, preparing drug-loaded alginate sulfate embolization microspheres. The invention also discloses the high-drug-loading degradable alginate sulfate vascular embolization microspheres obtained by the preparation method of the high-drug-loading degradable alginate sulfate vascular embolization microspheres and application of the high-drug-loading degradable alginate sulfate vascular embolization microspheres in preparation of an embolization agent for transcatheter arterial chemoembolization treatment. The alginate sulfate vascular embolization microspheres disclosed by the invention have good biocompatibility and are biodegradable, and the degradation product is safe and non-toxic. The preparation method has the advantages of simplicity and convenience in operation, high repeatability, mild conditions and the like, the prepared microspheres are uniform and controllable in particle size, and the vascular embolization microspheres prepared by the invention have good application prospects in the field of tumor interventional therapy.
Owner:OCEAN UNIV OF CHINA +1
Locally enhanced nickel-titanium alloy intracranial stent
InactiveCN110811735AImprove flexibilityImprove stabilityOcculdersMedical equipmentEmbolization Therapy
The invention relates to a locally enhanced nickel-titanium alloy intracranial stent, and belongs to the field of medical equipment. The intracranial stent can be used for assisting coil embolizationto treat intracranial aneurysms. An intracranial stent main body has a tubular structure formed by using single rhombus units composed of sine wave structures and connecting rods and double rhombus units each formed by combining two single rhombus units. The double rhombus units can improve the flexibility of the stent, and the single rhombus structures can guarantee the stability and supporting performance of the stent to a certain extent; and in addition, by adjusting the position and number of different-shape units, a plurality of stent structures can be designed and changed to achieve different mechanical characteristics. According to the locally enhanced nickel-titanium alloy intracranial stent design based on the mixed unit structure, an optimized unit ratio can be designed in different use environments, supporting enhancement at specific positions (such as a tumor path opening) can be realized on the premise that compliance of the stent is guaranteed, and the stent can be used for assisting the coil embolization.
Owner:INST OF METAL RESEARCH - CHINESE ACAD OF SCI
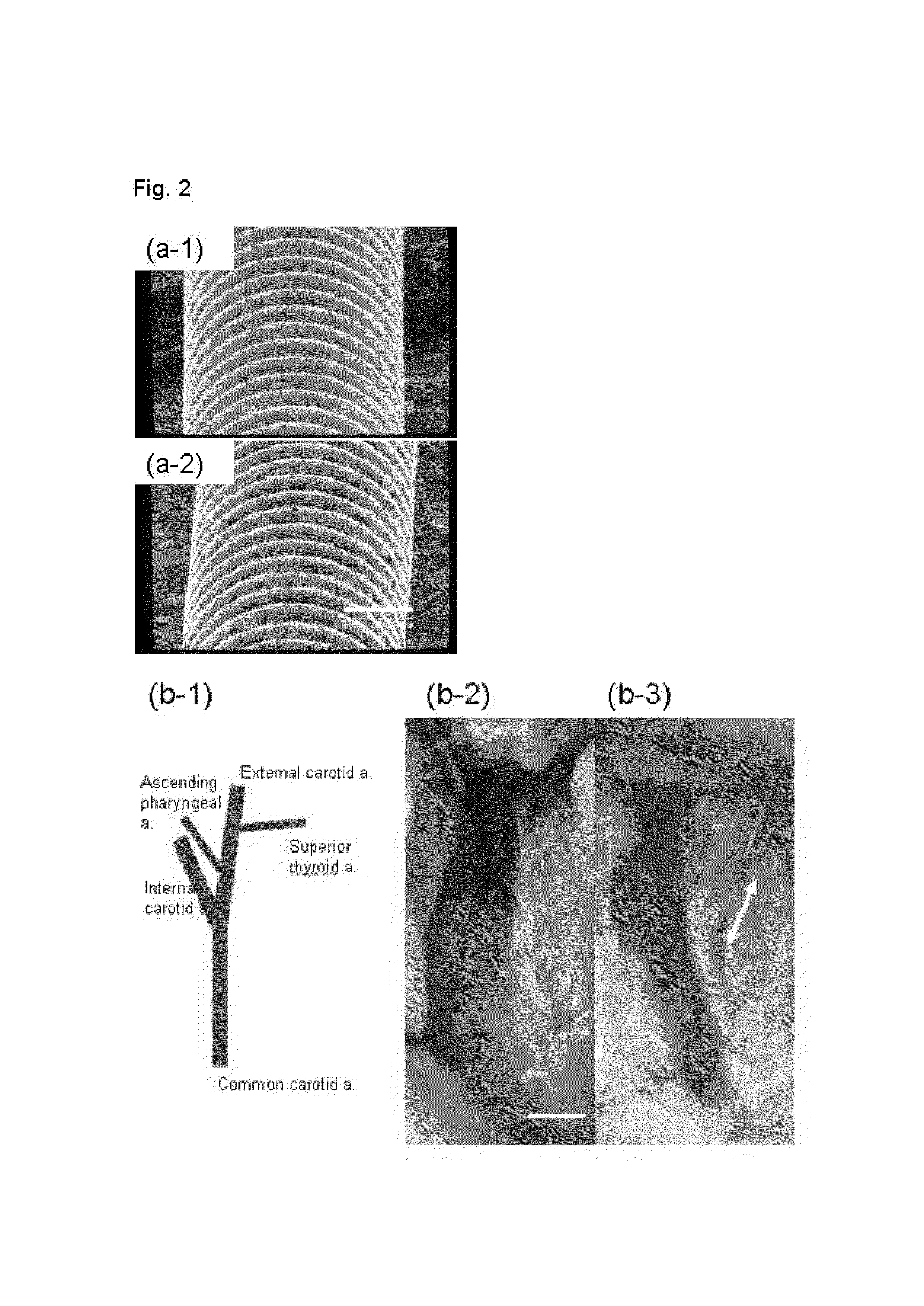
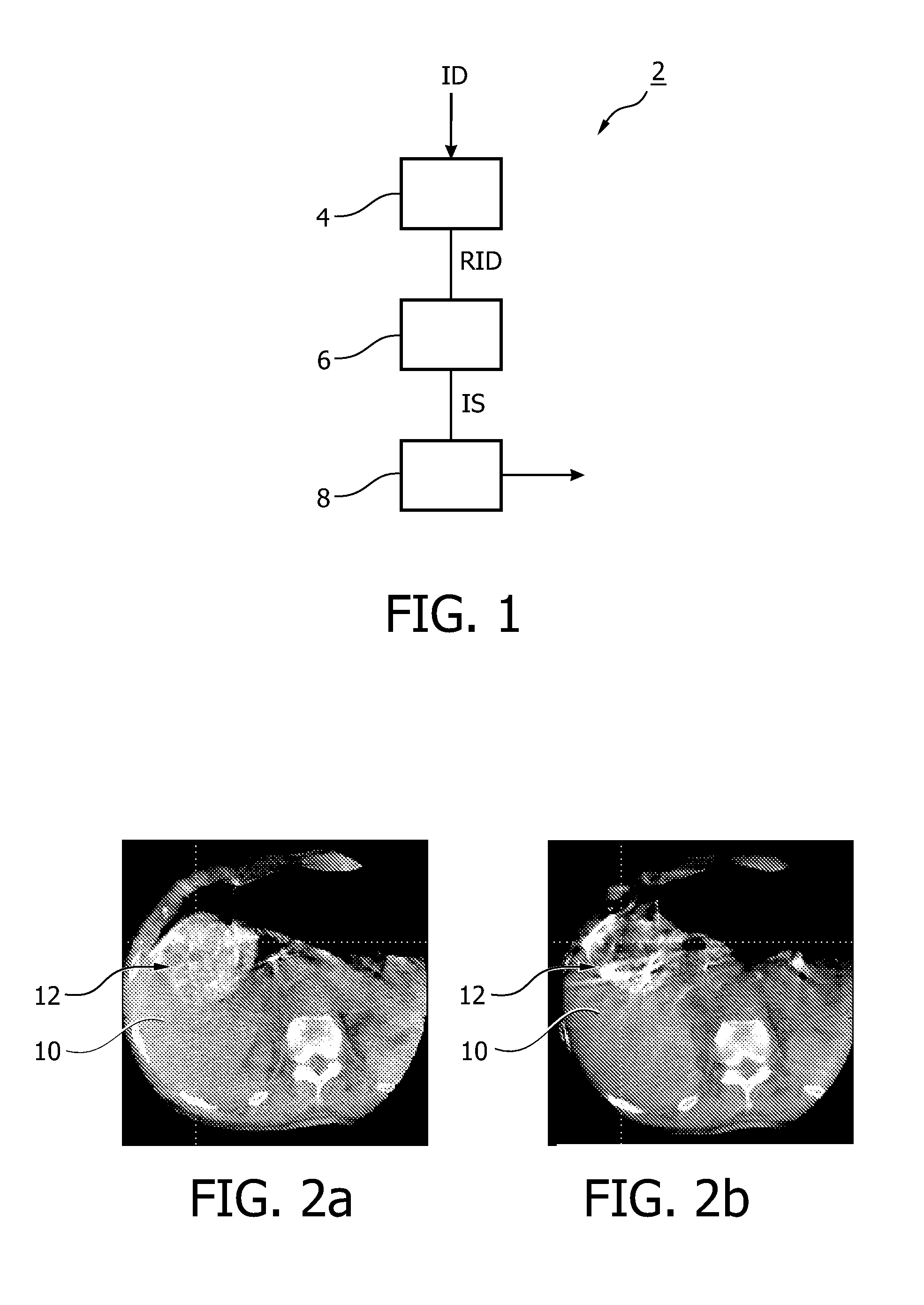
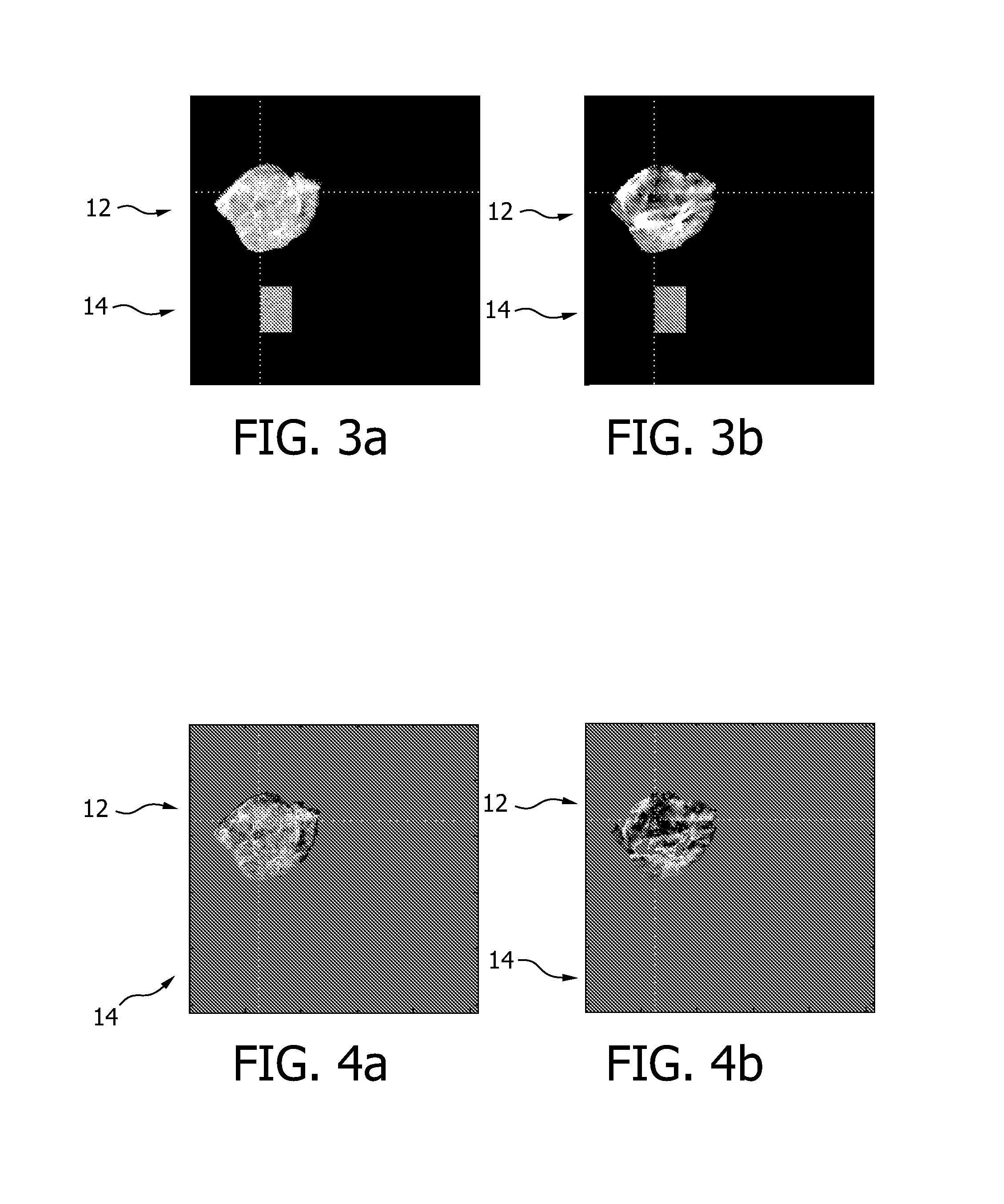
Automated quantification of intravascular embolization success
InactiveUS20130208957A1Increase awarenessReliable and preciseImage enhancementImage analysisEmbolization TherapyInterventional treatment
The present invention relates to a device (2) for automatically quantifying intravascular embolization success, comprising a registration unit (4) adapted for registering a first image and a second image, a segmentation unit (6) adapted for segmenting a tissue of interest in the first image and in the second image and an evaluation unit (8) for evaluating a deviation of perfusion of the tissue of interest by comparing the first image and the second image. The first image is obtained before an interventional treatment, whereas the second image is obtained after such a treatment. Evaluating may comprise comparing the segments of the first and the second images and thus providing a quantitative measure for a perfusion deviation of the tissue, e.g. the perfusion deviation of a tumorous tissue before and after an embolization treatment.
Owner:KONINKLIJKE PHILIPS ELECTRONICS NV
Porous embolization microspheres comprising drugs
PendingCN111741747ALess drugReduce lossesDiagnosticsGranular deliveryOrganic solventEmbolization Therapy
The current invention provides a method of forming polymeric microspheres loaded with a therapeutic agent, comprising: a. exposing porous polymeric microspheres to an organic solvent comprising a dissolved therapeutic agent thereby creating microspheres loaded with therapeutic agent, b. separating the microspheres loaded with therapeutic agent from the organic solvent, c. washing the microspheresloaded with therapeutic agent with water, and d. drying the washed microspheres. The microspheres are particularly useful in embolization therapy.
Owner:范里恩贝希尔有限公司
Embolism material for blood vessel, preparation method therefor and use thereof in preparation of drugs
ActiveUS20190247536A1Reduce viscosity of compositionLow viscositySurgical adhesivesPharmaceutical delivery mechanismEmbolization TherapyRadiology
Provided are the use of poly(N-isopropylacrylamide-co-butyl methacrylate) in preparing an embolism material for blood vessels, an embolism material for blood vessels and the use thereof in preparation of drugs. The embolism material for blood vessels comprises poly(N-isopropylacrylamide-co-butyl methacrylate) and a dispersion medium consisting of an electrolyte, a contrast agent, a pH regulator and water. The concentrations of the polymer, electrolyte and contrast agent are respectively 5-30 mg / ml, 0.1-30 mg / ml and 100-350 mg / ml based on iodine. The embolism material for blood vessels is suitable for embolization therapy of tumors in hypervascular and parenchymal visceral organs.
Magnetic resonance imaging detectable liquid embolism composition and its preparation and application
ActiveCN103536972BGood curative effectImprove securitySurgeryPharmaceutical delivery mechanismEmbolization TherapyX-ray
Owner:HYGEA MEDICAL TECH CO LTD
Liver-targeting platinum anticancer drug and synthetic method thereof
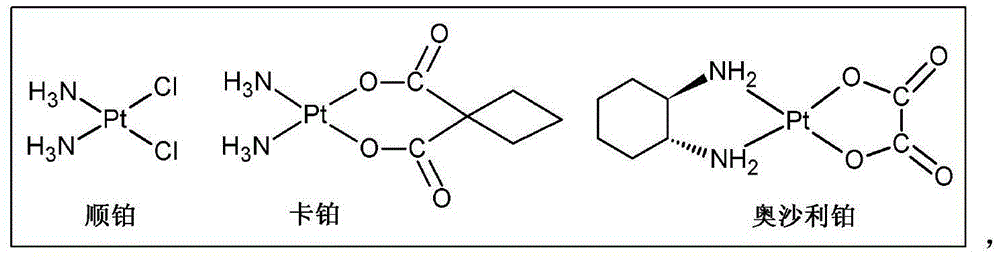
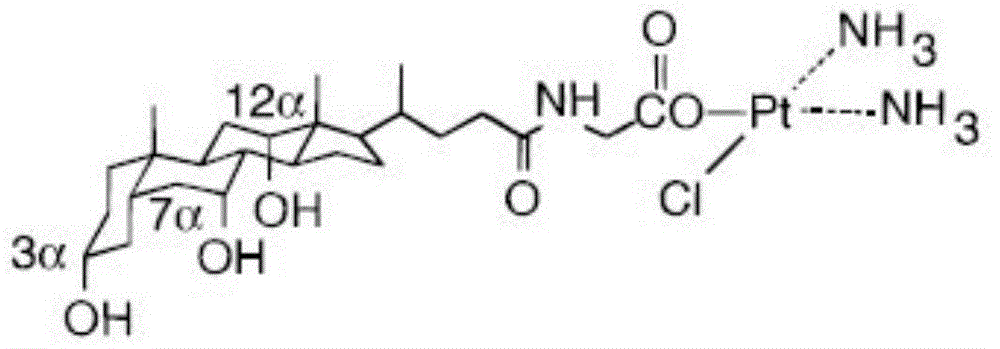
ActiveCN104610415AImprove stabilityIncrease fat solubilityPlatinum organic compoundsSteroidsEmbolization TherapyHyodeoxycholic acid
Owner:KUNMING INST OF PRECIOUS METALS +1
Absorbable tubular stent and preparation method and application thereof
InactiveCN105214144AHigh mechanical strengthGood grip rebound performanceSurgeryUrethral stentsEmbolization Therapy
The invention discloses an absorbable tubular stent. The absorbable tubular stent is characterized in that the tubular stent is made by blending of polylactic acid and acylated chitosan, and the wall of the tubular stent is provided with or without a porous structure or pattern structure. Due to addition of the acylated chitosan, local acidity generated by the polylactic acid in degradation can be regulated, and oligosaccharides and monosaccharide obtained by degradation of the acylated chitosan can be absorbed and used by organisms, so that high biocompatibility, degradability and absorbability are achieved; the tubular stent has excellent mechanical strength and press resilience. The absorbable tubular stent can be planted in vivo by surgeries to serve as an intravascular stent and is applicable to treatment of embolism or blood vessel lumina narrowing caused by vascular diseases; the absorbable tubular stent can also serve as a biliary stent to be applied to treatment of cholestasis and pancreaticobiliary obstruction caused by pancreatobiliary neoplasms; the absorbable tubular stent can further serve as a urethral stent to be applied to treatment of dysuria resulted from urethral compression caused by prostatic hyperplasia. The absorbable tubular stent can be degraded and absorbed in vivo finally along with lesion repair, in-vivo long-term retention is avoided, and the absorbable tubular stent has a promising market prospect.
Owner:OCEAN UNIV OF CHINA
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com