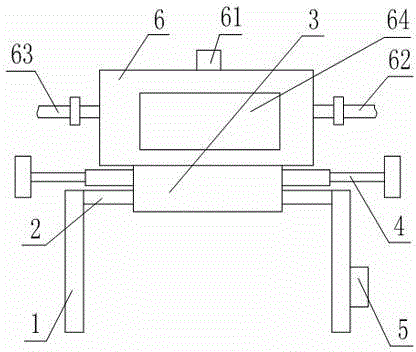
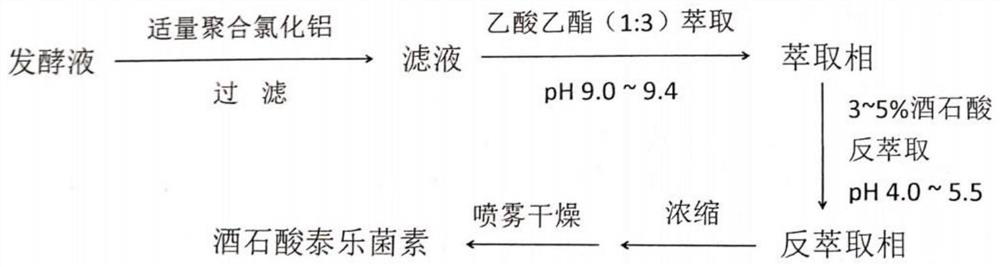
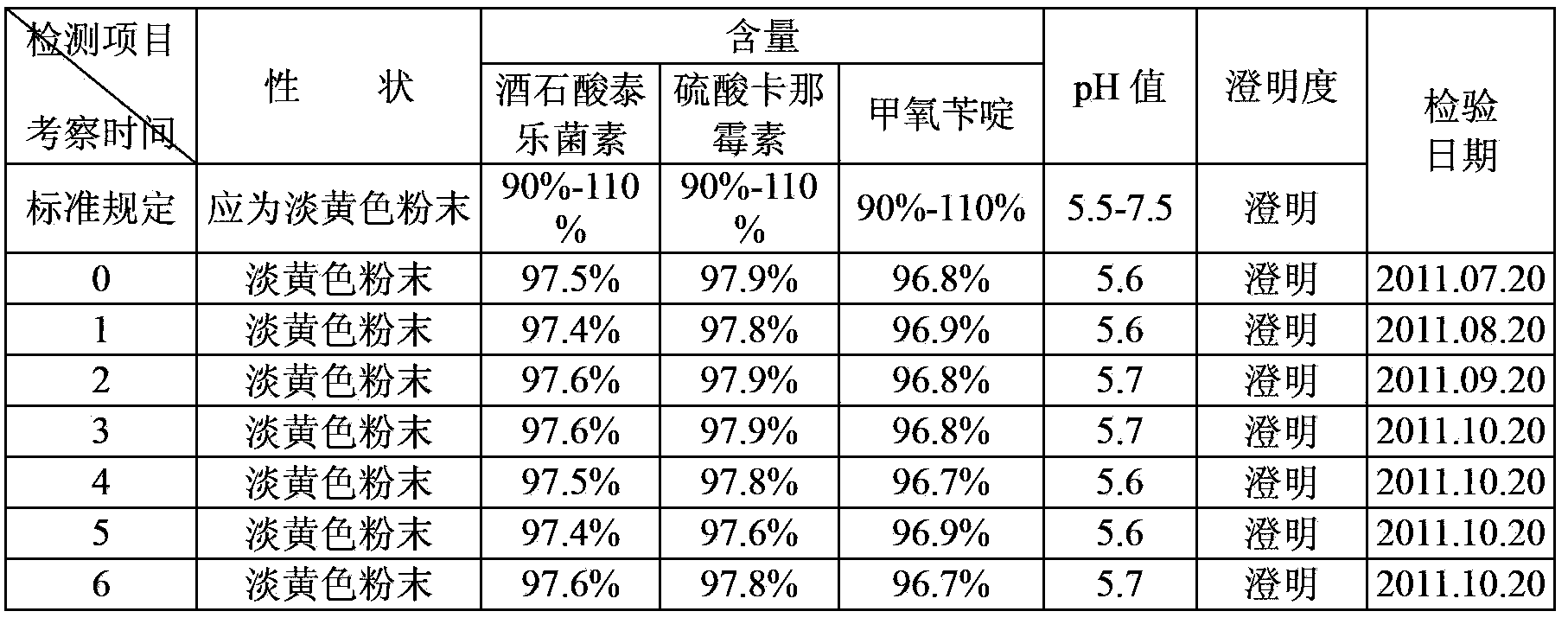
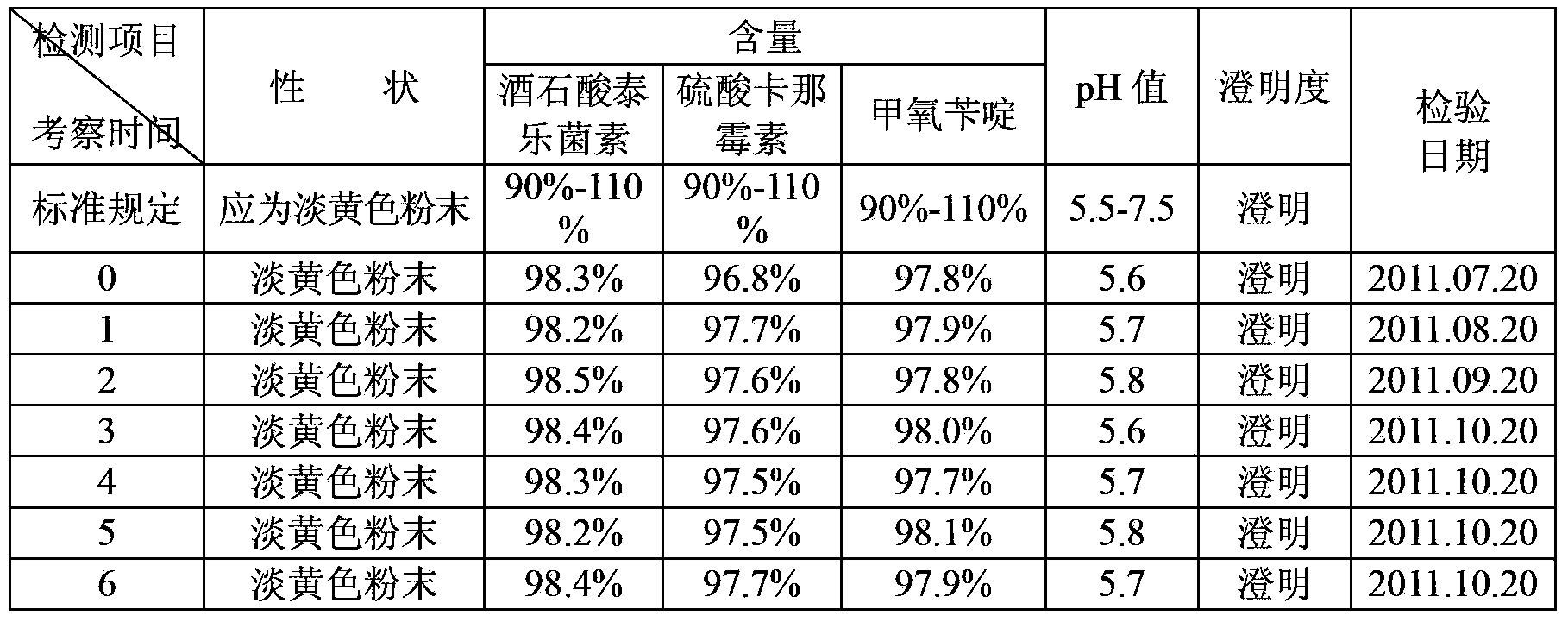
Patents
Literature
78 results about "TYLOSIN TARTRATE" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Tylosin tartrate is a particular type of antibiotic that is commonly used in a variety of animals, including dogs, cats, cattle, horses and more. This antibiotic serves a wide range of different purposes, but like other antibiotics it is designed specifically to target and kill certain types of bacteria.
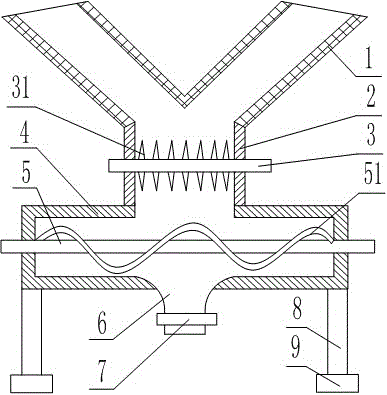
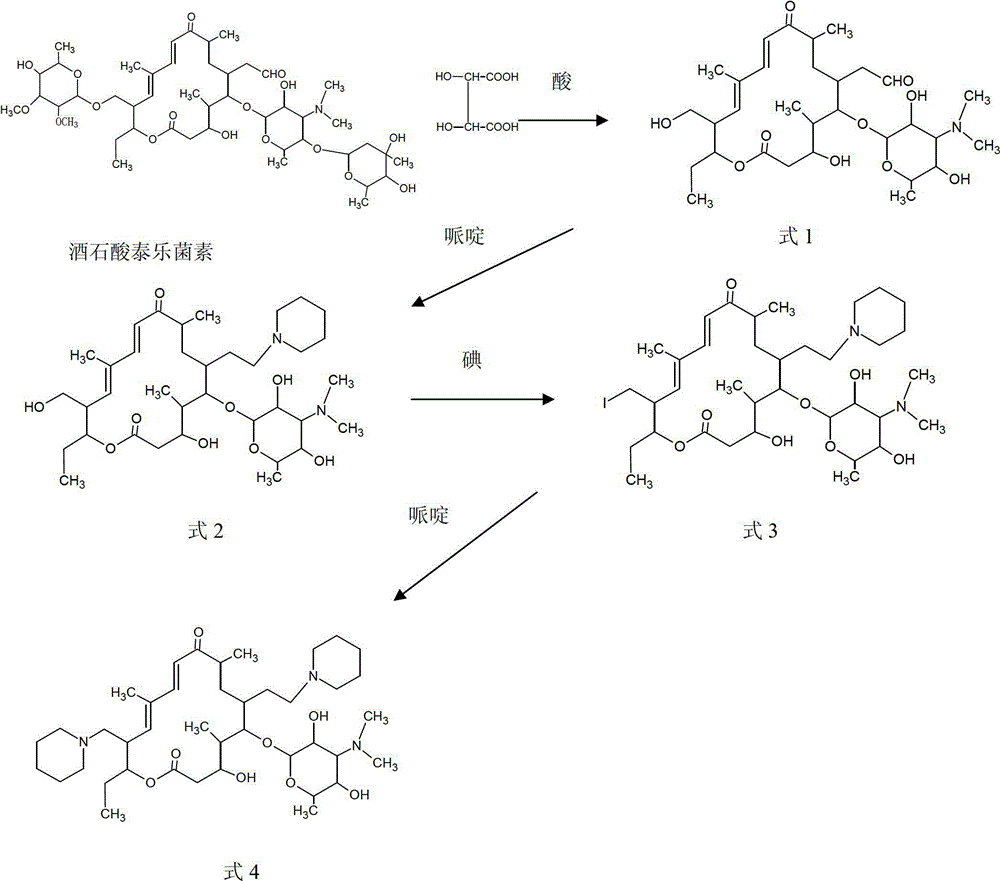
Process for preparing 20,23-bi-piperidyl-5-O-carbon mould amine glycosyl-tylosin lactone
ActiveCN102863487AOvercoming rare and expensive shortcomingsReduce manufacturing costSugar derivativesSugar derivatives preparationOrganic solventIodine
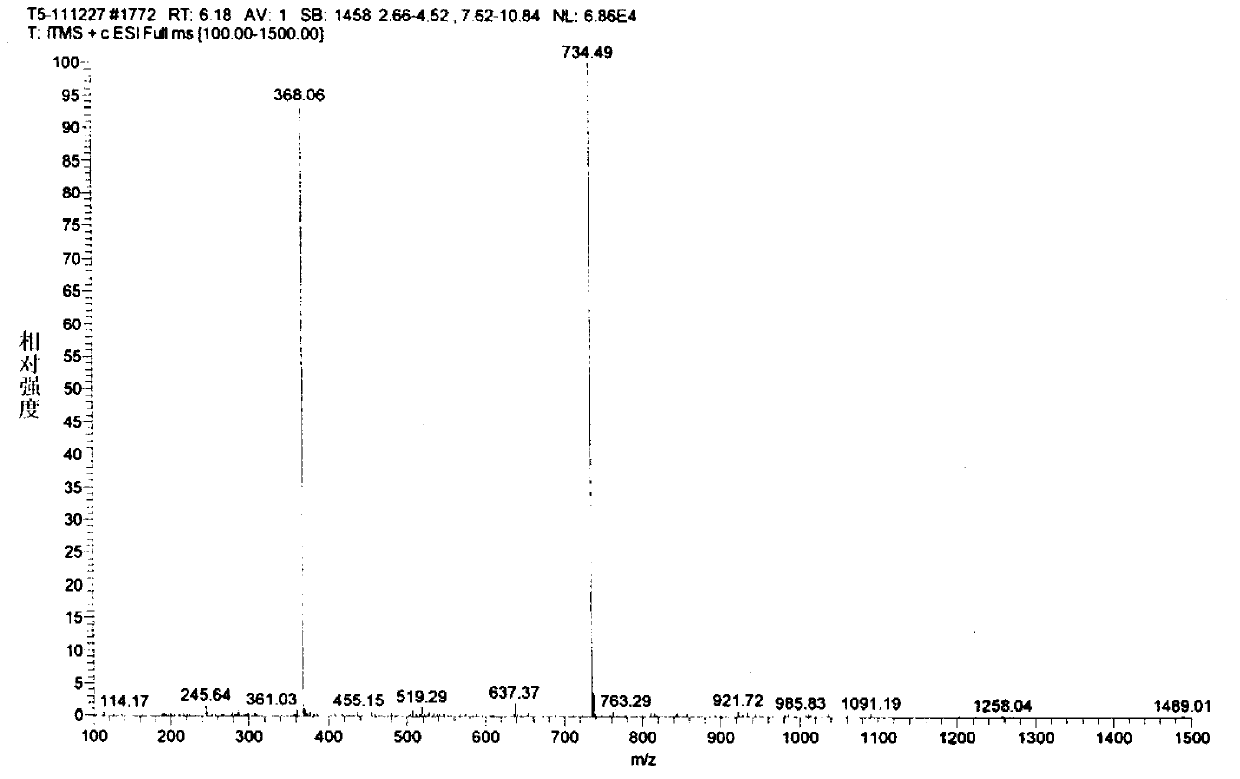
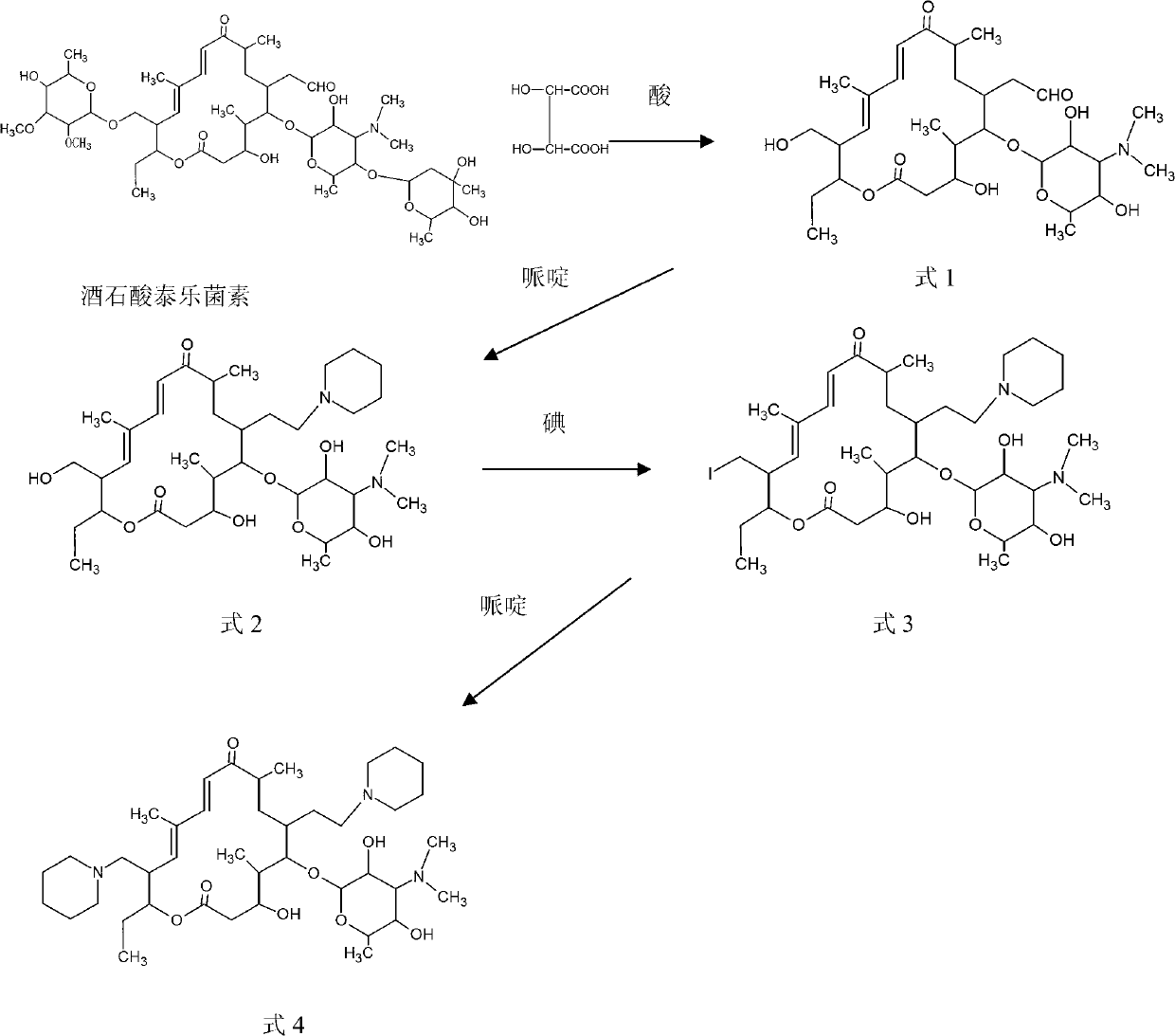
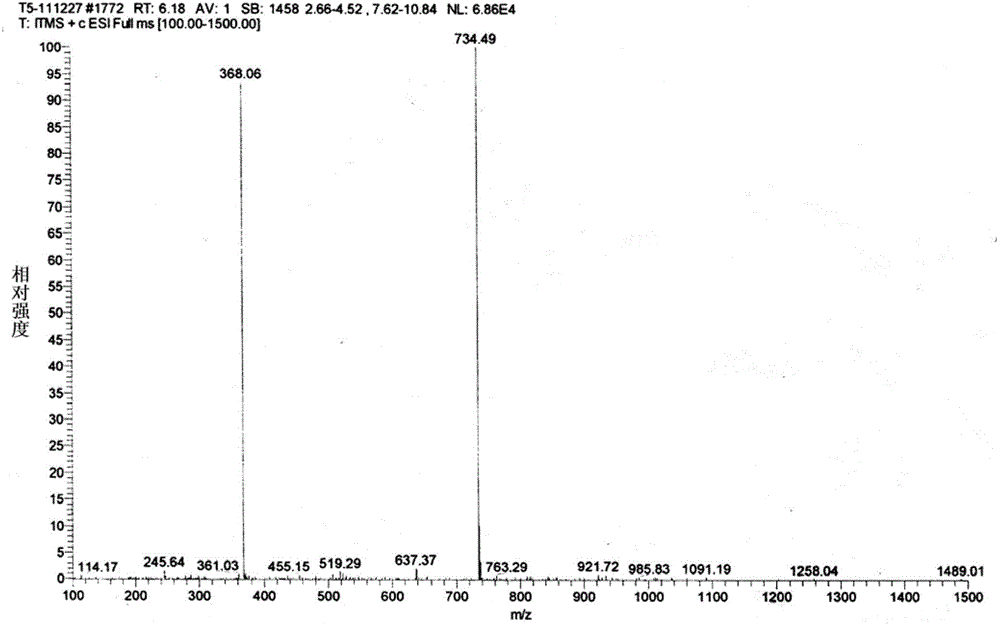
The invention relates to a process for preparing 20,23-bi-piperidyl-5-O-carbon mould amine glycosyl-tylosin lactone. Tylosin tartrate serves as a raw material, an intermediate product of 23-oxhydryl-5-O-carbon mould amine glycosyl-tylosin lactone is obtained through hydrolyzation, an organic phase is extracted through phase inversion, and an intermediate product of 20-piperidyl-23-oxhydryl-5-O-carbon mould amine glycosyl-tylosin lactone is produced through combination with piperidine under the effect of methanoic acid. A final product of the 20,23-bi-piperidyl-5-O-carbon mould amine glycosyl-tylosin lactone is formed by ammonization with the piperidine through iodination. By the aid of the process, the production technology is simplified, the dosage of auxiliary raw materials such as the piperidine, iodine and an organic solvent is greatly reduced, the purity of the obtained product is higher than 98%, the yield of the final product of the 20,23-bi-piperidyl-5-O-carbon mould amine glycosyl-tylosin lactone reaches 58.7%, and the process has good industrial application prospects.
Owner:QILU ANIMAL HEALTH PROD
Purification method of super tylosin
ActiveCN101381756AReduce pollutionSave organic solventSugar derivativesMicroorganism based processesMicroorganismPurification methods
The invention relates to a method for extracting super-tylosin from fermentation products of microorganism. The method adopts the secondary crystallization method, abandons the organic solvent extraction method in the prior art, can obtain products the purity of which is equivalent to that of extract of the solvent extraction method while simultaneously reducing the environmental pollution, simplifying the purification step and reducing the production cost, and is more suitable for industrial production. The products prepared by the method can be taken as bulk drugs of super-tylosin tartrate.
Owner:CHINA ANIMAL HUSBANDRY IND
Soluble powder for treating respiratory illness of livestock and poultry
InactiveCN101468032ASolve the problem that easily makes the body develop drug resistancePromote oral absorptionPowder deliveryOrganic active ingredientsDiseaseTrimethoprim
The invention relates to soluble powder for treating respiratory diseases of poultry, comprising tylosin tartrate, trimethoprim and auxiliary materials. As an antibiotic medicine, the inventive medicament is mainly used to prevent and treat various respiratory tract infections and chronic respiratory diseases of poultry. The tylosin tartrate and the trimethoprim have compatibility when used together. The soluble powder simultaneously acts on different sensitive organisms and mycoplasma, solves the problem of medicament tolerance caused by solely using tylosin to treat, has good absorption and relatively high safety when orally taken.
Owner:TIANJIN RINGPU BIO TECH
Tylosin tartrate or tylosin phosphate extraction from reextraction liquid
The invention improves a spray drying step in the tylosin production process in the prior art. The improvements are as follows: extracting tylosin tartrate or tylosin phosphate into an organic phase from a reextraction liquid (a water phase) by using trichloromethane or dichloromethane (an organic phase); then removing a solvent to obtain tylosin tartrate or tylosin phosphate; or after removal of most solvent, adding petroleum ether or ethyl acetate or butyl acetate into a concentrate, so as to precipitate the tylosin tartrate or tylosin phosphate. The method for the preparation of tylosin tartrate and phosphate tylosin has lower energy consumption and higher product yield than a spray drying dehydration method, and can increase the output value by more than 10 thousand yuan calculated by production of per ton of products.
Owner:中农华威制药股份有限公司
Acetylisovaleryl tylosin tartrate granular preparation and preparation method thereof
ActiveCN106361707AEvenly dispersedWell mixedAntibacterial agentsOrganic active ingredientsSpray GranulationDispersity
The invention discloses an acetylisovaleryl tylosin tartrate granular preparation and a preparation method thereof. The acetylisovaleryl tylosin tartrate granular preparation comprises the following steps: (1) preparing, namely, respectively weighing acetylisovaleryl tylosin tartrate and a carrier auxiliary material according to a formula ratio for later use; (2) mixing, namely, heating to melt the carrier auxiliary material, uniformly stirring, mixing acetylisovaleryl tylosin tartrate with the molten carrier auxiliary material, shearing, uniformly stirring so as to obtain a uniform dispersed system, and preparing mixed liquid; and (3) performing condensation spraying pelletization, namely, pumping the mixed liquid of step (2) to a condensation spray granulation system, performing centrifugal spray pelletization so as to prepare acetylisovaleryl tylosin tartrate granules, cooling, screening by using an oscillation sieve being 20-120 meshes, and collecting, thereby obtaining a product. According to the acetylisovaleryl tylosin tartrate granules prepared by the invention, the main medicine is uniformly distributed, the granules are round and tidy, the flowability and the dispersity of the granular preparation can be improved, and the acetylisovaleryl tylosin tartrate granular preparation can be easily mixed with feed; in addition, the acetylisovaleryl tylosin tartrate granular preparation adopts a simple production process, and is good in smell masking effect and good in slow-release effect.
Owner:GUANGDONG WENS DAHUANONG BIOTECH
Tilmicosin phosphate preparation method
ActiveCN105837648ASimple production processShorten the production cycleSugar derivativesSugar derivatives preparationOrganic solventPhosphorylation
The invention relates to a tilmicosin phosphate preparation method. The preparation method comprises that through hydrolysis under acidic conditions, alkalization extraction, amination, phosphorylation with phosphoric pentoxide and a small amount of water, full stirring salification, centrifugation separation after solid precipitation and vacuum drying, tilmicosin phosphate is prepared from tylosin tartrate as a raw material. According to the preparation method, phosphoric pentoxide and a small amount of water are directly added into an amination liquid, the mixture undergoes a phosphorylation reaction and tilmicosin phosphate is precipitated from the organic solvent. The preparation method has a final mole yield of 95% or more and a simple process route, can be operated easily, is free of spray drying production equipment, has a low production cost and realizes recycle of an organic solvent.
Owner:HUBEI LONGXIANG PHARMA TECH CO LTD
Long-acting compound ceftiofur suspension injection and its preparation method
InactiveCN102397282AFacilitated releaseHigh speedAntibacterial agentsOrganic active ingredientsSuspending AgentsAntibacterial activity
The invention discloses a long-acting compound ceftiofur suspension injection and its preparation technology. According to the invention, the compound ceftiofur suspension injection is prepared by using ceftiofur hydrochloride and tylosin tartrate as main drugs with assistance of appropriate auxiliary drugs. The suspension contains 5-20% (W / V) of ceftiofur hydrochloride, 5-20% (W / V) of tylosin tartrate, 0.5-2% (W / V) of a suspending agent, 0.01-0.5% (W / V) of an anti-oxidant, 0.1-3% (W / V) of a dispersant, and injection oil which is added to 100%. In comparison with a ceftiofur single preparation, the long-acting compound ceftiofur suspension injection has a strong antibacterial activity for Gram-negative bacteria and simultaneously has a strong effect of inhibiting Gram positive bacteria and mycoplasma. In addition, the preparation method provided by the invention provides a feasible approach for extended reproduction, and can be used to minimize times of drug administration and save manpower and material resources.
Owner:RINGPU TIANJIN BIOLOGICAL PHARMA
Aivlosin injection for swine and preparation method thereof
InactiveCN102119921ALong elimination half-lifeGood solubilization effectAntibacterial agentsOrganic active ingredientsOil phaseTert butyl
The invention relates to an aivlosin injection for swine. Each 100ml of tylosin injection contains 20g of acetylisovaleryl tylosin tartrate, 20-35g of propylene glycol, 10-15g of N,N-dimethyl acetamide, 2-4g of benzyl alcohol, 0.1-1.0g of di-tert-butyl-4-methylphenol and balance of glyceryl triacetate. The preparation method comprises the following steps of: mixing the glyceryl triacetate, the propylene glycol, the benzyl alcohol and the N,N-dimethyl acetamide evenly to obtain an oil phase; adding an antioxidant to the oil phase and stirring until the added particles are dissolved; and adding the aivlosin and stirring to dissolve. The aivlosin injection for swine adopts the oily solvent as a solvent, the medicament is dissolved in the oily solvent, and the elimination half life of the medicament in an animal body is prolonged by the characteristic of slow elimination of the oily solvent after intramuscular injection.
Owner:武汉回盛生物科技股份有限公司
Preparation method of tylosin phosphate or tartrate crystal
InactiveCN103275155AAvoid pollutionReduce pollutionSugar derivativesSugar derivatives preparationDispersityDissolution
The invention relates to a preparation method of tylosin phosphate or a tartrate crystal. The method comprises the steps that tylosin tartrate or phosphate powder is sufficiently dissolved in ethyl acetate; butyl acetate is slowly and dropwise added in a stirring state and at 0-5 DEG C, and is sufficiently mixed; the stirring state is kept for 1-2h after the adding is completed; centrifugal separation is performed; and the obtained crystal is subjected to drip washing by cold butyl acetate, and then subjected to vacuum drying until the drying loss is less than 1%. According to the preparation method, tylosin phosphate or tartrate is prepared into crystalline particles, and the crystalline particles replace powder; the specific gravity of the crystalline particles is greater, so that dust pollution is avoided in packaging and using processes; the particles are added to animal drinking water and can sink into the water bottom easily; the particles are better in dispersity, faster in dissolution rate and higher in content, can be directly added to animal feed or the animal drinking water; a solvent is very easy to recover; and the preparation method has a certain application prospect in a production process.
Owner:宁夏泰瑞制药股份有限公司
Compound acetylisovalery tylosin tartrate pellet and preparation method thereof
InactiveCN103271931AReduce usageMask bad smellAntibacterial agentsOrganic active ingredientsLactoseDrug release
The invention discloses a compound acetylisovalery tylosin tartrate pellet. The compound acetylisovalery tylosin tartrate pellet is made by wrapping a drug-loading pellet core by adopting a coating material, wherein the drug-loading pellet core comprises the following components in percentage by weight: 1-30% of acetylisovalery tylosin tartrate, 1-30% of sulfadimidine, 5-20% of lactose, 5-25% of corncob powder, 10-60% of starch, 15-35% of microcrystalline cellulose and 0.5-12% of pelletizing binding agent; and weight of the coating material is not more than 8% of the total weight of the compound acetylisovalery tylosin tartrate pellet. The compound acetylisovalery tylosin tartrate pellet has a better clinical treatment effect, application amount of acetylisovalery tylosin tartrate can be reduced, and production cost can be saved; and drug release can be delayed, drug action time can be prolonged, and long-acting effect can be achieved, so that administration frequency is reduced and labour intensity can be alleviated.
Owner:GUANGDONG WENS DAHUANONG BIOTECH
Preparation method of tylosin tartrate-doxycycline hydrochloride pharmaceutical composition
ActiveCN101744831AMask bitternessIncrease intakeAntibacterial agentsTetracycline active ingredientsOrganic solventAdjuvant
The invention discloses a preparation method of tylosin tartrate-doxycycline hydrochloride pharmaceutical composition, which comprises the following steps: dissolving a high molecular material in an organic solvent to prepare a dispersion medium; respectively adding tylosin tartrate powder and doxycycline hydrochloride powder to the dispersion medium slowly, heating, stirring, dissolving and then cooling to respectively obtain a tylosin tartrate microsphere suspension and a doxycycline hydrochloride microsphere suspension; and centrifuging, filtering, washing and drying the dispersion microsphere suspension to obtain microspheres. The weight ratio of the tylosin tartrate microspheres to the doxycycline hydrochloride microspheres is 3-4:1 counted by tylosin to doxycycline, and the microspheres are mixed uniformly and adjuvant is added to prepare soluble powder, particles and oral liquid. The pharmaceutical composition prepared in the invention has good curative effect, covers up the bitterness of the medicine, and is favorable for livestock and fowl to take.
Owner:PU LIKE BIO ENG
Compound doxycycline hydrochloride composition for preventing and treating colibacillosis and chronic respiratory disease of poultry as well as preparation method thereof
InactiveCN102813909AReasonable compositionEasy to makeAntibacterial agentsTetracycline active ingredientsEscherichia coliDisease
The invention relates to a compound doxycycline hydrochloride composition for preventing and treating colibacillosis and chronic respiratory disease of poultry as well as a preparation method of the compound doxycycline hydrochloride composition. The compound doxycycline hydrochloride composition is prepared by uniformly mixing the following components by weight: 10-15 parts of doxycycline hydrochloride, 10-20 parts of colistin sulfate, 5-15 parts of tylosin tartrate, 5-10 parts of trimethoprim lactate and 40-70 parts of anhydrous dextrose. The compound doxycycline hydrochloride composition has the beneficial effects of reasonable formula, easiness in preparation, remarkable treatment effect and clinically proven special effect for treating colibacillosis and chronic respiratory disease of the poultry.
Owner:QINGDAO LVMAN BIOLOGICAL ENG
Powder for treating livestock and poultry mycoplasma infection
InactiveCN101450065AControl secondary infectionControl mixed infectionOrganic active ingredientsPowder deliveryOral medicationSulfanilamide
The invention relates to a powder agent for treating livestock and poultry mycoplasma infection, comprising tylosin tartrate, sulfadimidine sodium and auxiliary materials. The medicament of the invention can be mainly used for preventing and treating the mycoplasma disease of the livestock and poultry, meanwhile the sulfadimidine sodium is added in the preparation for controlling the secondary infection mixed infection caused by the mycoplasma, the medicament has good oral administration absorption effect and high safety, can be suitable for popularization and application in the animal production.
Owner:TIANJIN RINGPU BIO TECH
Tylosin tartrate premix composition and preparation method of tylosin tartrate sustained-released pellets
InactiveCN106309409AAchieve full encapsulation rateHigh parcel rateAntibacterial agentsOrganic active ingredientsSolid componentAdhesive
The invention relates to a tylosin tartrate premix composition and a preparation method of tylosin tartrate sustained-released pellets, aiming at solving the problems in the prior art that the encapsulation efficiency of tylosin tartrate is low, the light-shielding property and the palatability are poor, the releasing efficiency of the tylosin tartrate cannot be effectively controlled and industrial production is not easy to realize and the like. The preparation method comprises: firstly crushing a solid component and sieving; mixing the tylosin tartrate and a filling agent; adding an adhesive and covering an enteric coating layer and a sustained-released layer on the tylosin tartrate through a granulator. According to the tylosin tartrate premix composition and the preparation method of the tylosin tartrate sustained-released pellets, provided by the invention, whole encapsulation is realized and the palatability is good; the releasing efficiency of the tylosin tartrate can be effectively controlled and the industrial production can be realized.
Owner:乐山市佰尔特生物工程合伙企业(有限合伙)
Method for preparing high-quality tilmicosin through low-quality tylosin
InactiveCN105777828ALower quality requirementsLow costSugar derivativesSugar derivatives preparationHydrolysateProduct base
The invention belongs to the technical field of pharmaceuticals, and discloses a method for synthesizing tilmicosin through low-quality tylosin. The method comprises the steps that tylosin tartrate and 3,5-dimethylpiperidine are subjected to an amination reaction by taking isoamyl acetate as solvent under the certain acidity and temperature conditions, acidic hydrolysis is conducted, hydrolysate passes through a Beta molecular sieve, impurities produced by a D component in tylosin are adsorbed, alkali adjustment and crystallization are conducted, and high-quality tilmicosin is obtained. According to the method for synthesizing tilmicosin through low-quality tylosin, low-quality tylosin tartrate can be adopted, the quality requirement of the raw material is lowered, the raw material cost is reduced, the applied Beta molecular sieve is easy to separate and capable of being recycled, economical efficiency and environmental protection are achieved, operation is easy, the method is suitable for industrial production, and the quality of the obtained product is superior to that of the product based on Pharmacopeia 2010.
Owner:HEZE CITY FANGMING PHARMA
Method for recrystallizing tylosin tartrate
ActiveCN107903294AImprove solubilityModerate volatilitySugar derivativesSugar derivatives preparationPhase splittingSec-Butyl acetate
The invention relates to a method for recrystallizing tylosin tartrate. The method comprises the following process steps: firstly extracting a tylosin tartrate refined solution by using dichloromethane, slowly and dropwise adding sec-butyl acetate in a dichloromethane phase obtained after phase splitting until separating out crystal; then centrifuging and drying the crystal. According to the characteristic that the tylosin tartrate can be dissolved in the dichloromethane but not be dissolved in the sec-butyl acetate, the tylosin tartrate refined solution is subjected to recrystallization treatment by adopting a dichloromethane extracting and sec-butyl acetate crystallization separation method; the yield of a product obtained through the method disclosed by the invention is up to 97.0 percent or more, and the content of a tylosin component A is up to 94.0 percent or more; the method has the characteristics that technological treatment is simple, no pollution is generated, the cost is low, the period is short, the yield of the obtained product is high, the content of the tylosin component A is high, the quality is high, and the like.
Owner:NINGXIA TAIYICIN BIOTECH CO LTD
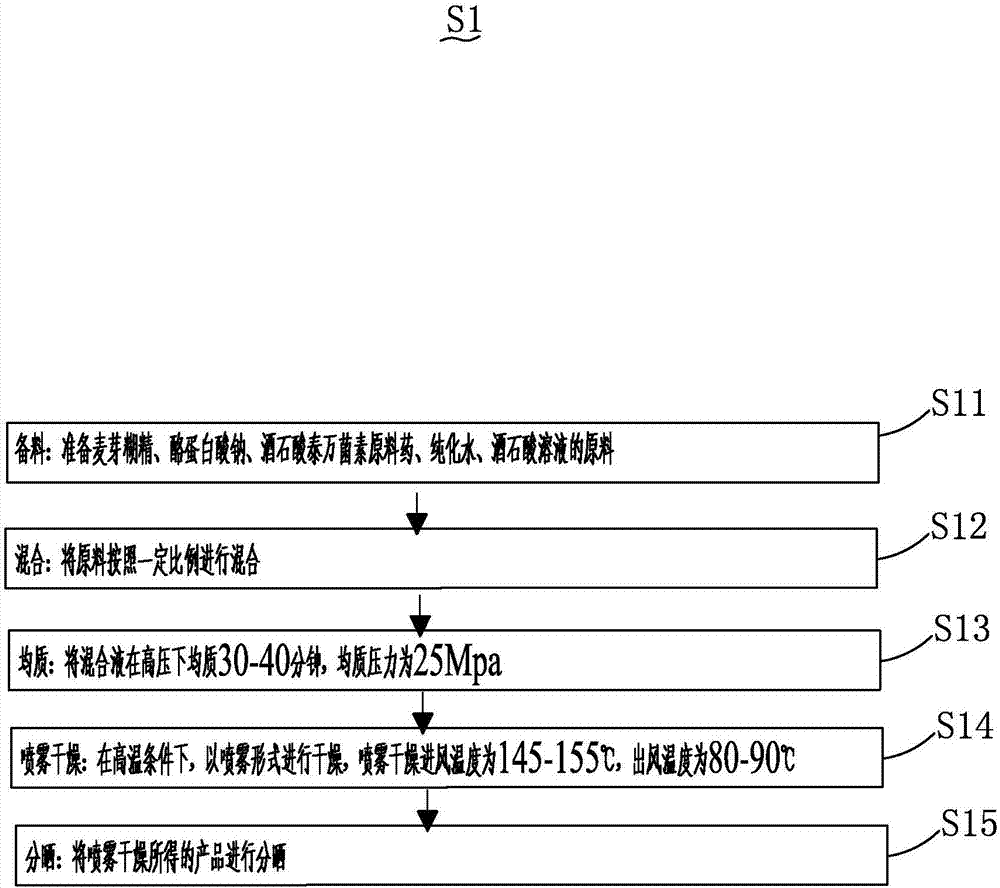
Preparation technology of tylosin tartrate premix based on uniform mixing effect improvement
InactiveCN105640892AContinuous mixing outputImprove uniformityAntibacterial agentsPowder deliveryAdditive ingredientDistillation
The invention discloses a preparation technology of a tylosin tartrate premix based on uniform mixing effect improvement. The preparation technology includes the steps of 1), extracting tylosin tartrate, namely adopting trichloromethane for extracting reextraction liquid containing the tylosin tartrate, and subjecting the trichloromethane extraction liquid to anhydrous sodium sulfate to dehydration and reduced pressure distillation to remove the trichloromethane completely so as to obtain tylosin tartrate; 2) drying the extracted tylosin tartrate and preparing the dried tylosin tartrate into powder; 3) preparing the tylosin tartrate premix by uniformly mixing the tylosin tartrate powder with minor ingredients through a mixing device, and bagging according to specifications. The preparation technology of the tylosin tartrate premix based on uniform mixing effect improvement has the advantages that since a V-shaped feeding port allowing simultaneous addition of two different raw materials is arranged, the fed raw materials pass through a primary mixing chamber and a secondary mixing chamber sequentially to be subjected to two-time mixing, and accordingly raw material mixing uniformity is improved, the uniformly-mixed raw materials are discharged out of a discharging port, and continuous uniform mixing and outputting of active pharmaceutical ingredients are achieved.
Owner:CHINA CHENGDU ANIMAL HUSBANDRY IND BIOPHARM
Preparation technology of tylosin tartrate for injection
InactiveCN104892705ADissolve fastFall off quicklyAntibacterial agentsOrganic active ingredientsDistillationNitrogen
The present invention relates to a preparation technology of tylosin tartrate for injection. The preparation technology comprises the following steps of: tylosin tartrate extraction: extracting by adopting chloroform to obtain an extraction liquid of an aqueous phase liquid and chloroform, sequentially performing dehydration by using anhydrous sodium sulfate and distillation at a reduced pressure on the extraction liquid of chloroform to eliminate the chloroform completely so as to obtain the tylosin tartrate; and drying the extracted tylosin tartrate and preparing the dried tylosin tartrate into powder. The preparation technology of the tylosin tartrate further comprises the following steps of: cleaning a reagent bottle, wherein the reagent bottle is immersed into a hydrochloric acid solution with condensation of 0.01mol / L-0.1mol / L; then subjecting the mixture to ultrasonic cleaning, spray-type flushing, drying and sterilization; packaging a certain amount of the tylosin tartrate powder to the cleaned reagent bottle according to specifications; inflating the reagent bottle with nitrogen; covering the bottleneck with a pre-treated rubber plug; performing cover-piercing treatment by using an aluminum cover. The preparation technology of tylosin tartrate for injection disclosed by the invention overcomes the problem that the existing preparation technology of tylosin tartrate for injection causes a relatively low production speed of the tylosin tartrate for injection.
Owner:CHINA CHENGDU ANIMAL HUSBANDRY IND BIOPHARM
Acetylisovaleryl Tylosin Tartrate clathration enteric-coated preparation and preparing method thereof
ActiveCN108261410AGuaranteed stabilityGood water solubilityAntibacterial agentsOrganic active ingredientsTYLOSIN TARTRATEEnteric coated
The invention provides a preparing method of an Acetylisovaleryl Tylosin Tartrate clathration enteric-coated preparation. The preparing method comprises the steps of clathration of Acetylisovaleryl Tylosin Tartrate in hydroxypropyl-beta-cyclodextrin, Acetylisovaleryl Tylosin Tartrat is directly used for being manufactured into a medicine carrying inner core, and then the medicine carrying inner core is covered with an isolating layer and a coating layer separately to prepare the Acetylisovaleryl Tylosin Tartrate clathration enteric-coated preparation. Compared with the prior art, the coating and pelletizing technology of Acetylisovaleryl Tylosin Tartrate is implemented at a time, the operation is simple, and the production cost is saved. Meanwhile, the influence generated by gastric acid is avoided, the preparation can be targetedly released in the intestinal part, and the clinical treatment effect is better.
Owner:武汉回盛生物科技股份有限公司
Preparation process avoiding the reduction of tylosin tartrate original drug potency
InactiveCN104926899AAvoid the problem of reduced potency of the original drugAvoid performance lossSugar derivativesSugar derivatives preparationTemperature controlDistillation
The invention relates to a preparation process avoiding the reduction of tylosin tartrate original drug potency. The method comprises the following steps: tylosin tartrate is extracted, wherein a back extraction liquid containing tylosin tartrate is extracted with trichloromethane, such that water-phase liquid and a trichloromethane extraction liquid are obtained; the trichloromethane extraction liquid is dehydrated with anhydrous sodium sulfate and is subjected to reduced-pressure distillation, such that trichloromethane is removed, and tylosin tartrate is obtained; the extracted tylosin tartrate is dried and prepared into powder; the tylosin tartrate is powder is well mixed with auxiliary materials according to a certain ratio in a shaking bed with a temperature control function; reagent bottles are cleaned; the tylosin tartrate powder is added into the reagent bottles according to a specified amount, and the bottles are filled with nitrogen gas; the bottles are covered by pretreated rubber plugs; and the plugs are capped with aluminum caps. With the process provided by the invention, a problem of injection-use tylosin tartrate original drug potency reduction caused by existing tylosin tartrate preparation processes is overcome.
Owner:CHINA CHENGDU ANIMAL HUSBANDRY IND BIOPHARM
Preparation method of tylosin tartrate microspheres
ActiveCN101947207AHigh drug loadingHigh encapsulation efficiencyAntibacterial agentsOrganic active ingredientsMicrosphereOil phase
The invention discloses a preparation method of tylosin tartrate microspheres. The method uses gelatin as the carrier, liquid paraffin and Span-80 as the oil phase, and glutaraldehyde as the crosslinking agent to prepare tylosin tartrate microspheres. The tylosin tartrate microspheres prepared by the invention use gelatin as the carrier material, have no adverse reactions and no immunogenicity, have biodegradability and wide application range and can be taken by mouth or through injection. liquid paraffin and Span-80 are used as the oil phase, thus increasing the drug-loading rate and the encapsulation rate, facilitating the crosslinking and curing of glutaraldehyde, prolonging the elimination half life of the microsphere medicine and performing drug controlled release in animals. In addition, the preparation method of the invention has simple operation, the adopted reagent is common, the preparation cost is lower and the method is easy to popularize and apply.
Owner:ZHENGZHOU HOUYI PHARMA
Preparation methods for preparing tylosin-containing encapsulated core material with tylosin stripping solution and micro-capsule of tylosin-containing encapsulated core material
InactiveCN107281158AHigh yieldReduce energy consumptionAntibacterial agentsOrganic active ingredientsOrganic solventAqueous solution
The invention discloses preparation methods for preparing corncob powder and tylosin (tylosin tartrate or tylosin phosphate)-containing encapsulated core material by directly combining a purified tylosin stripping solution (an aqueous solution containing the tylosin tartrate or the tylosin phosphate) with corncob powder and a micro-capsule of the encapsulated core material. By using the preparation methods provided by the invention to prepare the tylosin / corncob powder-containing encapsulated core material and the micro-capsule of the encapsulated core material, the yield is higher, and the energy consumption is lower; without using an organic solvent, the flammable and combustible risks and the dust pollution are lowered, the intermediate manufacturing cost and the unnecessary pollution are reduced, and a product can be directly used for clinical veterinary medicine.
Owner:中农华威生物制药(湖北)有限公司
Aqueous phase preparation method and application of tylosin tartrate surface molecularly imprinted polymer
ActiveCN109021171AUniform particle sizeLarge adsorption capacityOther chemical processesWater contaminantsFunctional monomerSorbent
Belonging to the technical field of sample pretreatment and pollutant analysis and detection, the invention discloses an aqueous phase preparation method and application of a tylosin tartrate surfacemolecularly imprinted polymer. The technical scheme includes the key points that: 2-acrylamide-2-methylpropane sulfonic acid and / or 1, 4-butanediyl-3, 3'-bis-1-vinyl imidazolium chloride can be adopted as the functional monomer, tylosin tartrate is taken as the template molecule, pure water is adopted as the preparation solvent, the crosslinking agent N, N'-methylene bisacrylamide and the initiator azobisisobutyronitrile are utilized for thermal initiation of polymerization reaction to prepare the tylosin tartrate surface molecularly imprinted polymer on a styrene-divinyl benzene granular carrier surface. The tylosin tartrate surface molecularly imprinted polymer has action sites for specific recognition of tylosin tartrate, and can be used for selective adsorption of trace tylosin tartrate in environmental samples. According to the invention, the preparation process is simple and the cost is low, and the polymer adsorbent material has uniform particle size, large adsorption capacity and high mass transfer rate.
Owner:HENAN NORMAL UNIV
Decolorization method of tylosin tartrate
PendingCN111620919AAchieve separationAchieve purificationSugar derivativesSugar derivatives preparationActivated carbonSeparation technology
The present invention provides a decolorization method of tylosin tartrate. The method includes the following steps: dissolving tylosin tartrate in water, first decolorizing with activated carbon, then decolorizing with silica gel, and finally using ultrafiltration membrane for decolorizing three times. This method uses a combination of physical adsorption technology and ultrafiltration membrane separation technology, can effectively remove pigments in tylosin tartrate, has good separation effect, thus can improve yield and purification effect of tylosin, and has technical advantages such as no destruction of active ingredients, no secondary pollution, repeated use of adsorbents and ultrafiltration membranes, and convenient operation.
Owner:NINGXIA TAIYICIN BIOTECH CO LTD
Preparation method of synergistic tylosin tartrate soluble powder compound medicine
InactiveCN103405463AWide range of indicationsGood curative effectAntibacterial agentsOrganic active ingredientsTrimethoprimSecondary Infections
The invention aims at providing a preparation method of a synergistic tylosin tartrate soluble powder compound medicine. The synergistic tylosin tartrate soluble powder compound medicine has a rapid and definite curative effect on respiratory system infections and complications of livestock and poultry. The preparation method comprises the following steps of: mixing 0.2 part of trimethoprim and 0.2 part of cosolvent, then crushing, and sieving by using a 120 meshes sieve or a finer sieve; and then uniformly mixing with 1 part of tylosin tartrate, 1 part of kanamycin sulfate and 0.05-0.1 part of pain alleviant by an equivalently successive increase method, thus obtaining the synergistic tylosin tartrate soluble powder compound medicine. The cosolvent is citric acid or succinic acid or the mixture of citric acid and succinic acid and the pain alleviant is procaine hydrochloride or lidocaine hydrochloride or the mixture of procaine hydrochloride and lidocaine hydrochloride. The animal synergistic tylosin tartrate soluble powder compound medicine for injection contains three medical components which are tylosin tartrate, kanamycin sulfate and trimethoprim, can be used for solving the problems that the secondary infection cannot be controlled very well during the treatment of mycoplasma infection due to the narrow antibacterial spectrum of the tylosin tartrate, and the trimethoprim is insoluble in water, has a synergistic effect on tylosin tartrate and kanamycin sulfate and has an obvious medicine curative effect.
Owner:四川联美生物药业有限公司
Compound emulsion for animals and preparation method thereof
InactiveCN102058612AReduce poisonReduce toxicity and reduce environmental pollutionAntibacterial agentsOrganic active ingredientsAzithromycinOrganic solvent
The invention belongs to the technical field of veterinary medicaments, and particularly relates to a compound emulsion for animals and a preparation method thereof. The compound emulsion for animals comprises tylosin tartrate, azithromycin, emulsifier, poloxamer, propylene glycol, soybean oil for injection and water for injection. The compound emulsion for animals has high safety; and because the emulsion contains no organic solvent, the toxicity of the organic solvent is reduced, and the emulsion has good environmental friendliness.
Owner:河南亚卫动物药业有限公司
Preparation method of tylosin tartrate premix
InactiveCN105853363AIncrease production speedContinuous mixing outputAntibacterial agentsPowder deliveryDistillationTYLOSIN TARTRATE
The invention discloses a preparation method of a tylosin tartrate premix. The method comprises the following steps: 1, extracting tylosin tartrate: extracting a tylosin tartrate-containing back extraction solution with trichloromethane, dehydrating the obtained trichloromethane extract solution through using anhydrous sodium sulfate, and carrying out reduced pressure distillation to completely remove trichloromethane in order to obtain tylosin tartrate; 2, drying the extracted tylosin tartrate, and processing the dried tylosin tartrate to obtain powder; and 3, preparing the tylosin tartrate premix: uniformly mixing the tylosin tartrate powder with auxiliary materials through a mixing device, and packaging the obtained premix in bags by specification. The problem of slow production speed of the tylosin tartrate premix, easily induced by present preparation technologies of the tylosin tartrate premix, is solved in the invention.
Owner:CHINA CHENGDU ANIMAL HUSBANDRY IND BIOPHARM
Process for preparing 20,23-bi-piperidyl-5-O-carbon mould amine glycosyl-tylosin lactone
ActiveCN102863487BOvercoming rare and expensive shortcomingsReduce manufacturing costSugar derivativesSugar derivatives preparationOrganic solventIodine
The invention relates to a process for preparing 20,23-bi-piperidyl-5-O-carbon mould amine glycosyl-tylosin lactone. Tylosin tartrate serves as a raw material, an intermediate product of 23-oxhydryl-5-O-carbon mould amine glycosyl-tylosin lactone is obtained through hydrolyzation, an organic phase is extracted through phase inversion, and an intermediate product of 20-piperidyl-23-oxhydryl-5-O-carbon mould amine glycosyl-tylosin lactone is produced through combination with piperidine under the effect of methanoic acid. A final product of the 20,23-bi-piperidyl-5-O-carbon mould amine glycosyl-tylosin lactone is formed by ammonization with the piperidine through iodination. By the aid of the process, the production technology is simplified, the dosage of auxiliary raw materials such as the piperidine, iodine and an organic solvent is greatly reduced, the purity of the obtained product is higher than 98%, the yield of the final product of the 20,23-bi-piperidyl-5-O-carbon mould amine glycosyl-tylosin lactone reaches 58.7%, and the process has good industrial application prospects.
Owner:QILU ANIMAL HEALTH PROD
Microcapsule type tylosin tartrate preparation and production technology thereof
PendingCN107412187AHigh embedding rateImprove antioxidant capacityAntibacterial agentsOrganic active ingredientsSodium CaseinateMaltodextrin
The invention provides a microcapsule type tylosin tartrate preparation and a production technology thereof. The microcapsule type tylosin tartrate preparation is prepared from maltodextrin, sodium caseinate, a crude tylosin tartrate drug, purified water and a tartaric acid solution. The microcapsule type tylosin tartrate preparation and the production technology thereof have the advantages that a preparation method is simple and feasible, the production efficiency is high, the produced microcapsule type preparation is high in encapsulation rate, good in anti-oxidation performance, slow to degrade, controllable in quality and the like.
Owner:内蒙古中牧生物药业有限公司
Soluble powder for treating respiratory diseases of livestock and poultry
InactiveCN103830254ASolve the problem that easily makes the body develop drug resistancePromote oral absorptionAntibacterial agentsPowder deliveryDiseaseTrimethoprim
Belonging to the technical field of veterinary medicines, the invention relates to a soluble powder for treating respiratory diseases of livestock and poultry. The soluble powder comprises tylosin tartrate, trimethoprim and auxiliary materials. As an antibiotic medicine, the medicine provided by the invention is mainly used for various respiratory tract infection and chronic respiratory diseases of livestock and poultry caused by mixed infection of sensitive bacteria and mycoplasmas. Tylosin tartrate and trimethoprim are adopted for compatible use to act on different sensitive bacteria and mycoplasmas at the same time, thus solving the drug resistance problem generated after single use of tylosin for treatment. The soluble powder provided by the invention has the advantages of good oral administration absorption and high safety.
Owner:QINGDAO KDN BIOTECH
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com