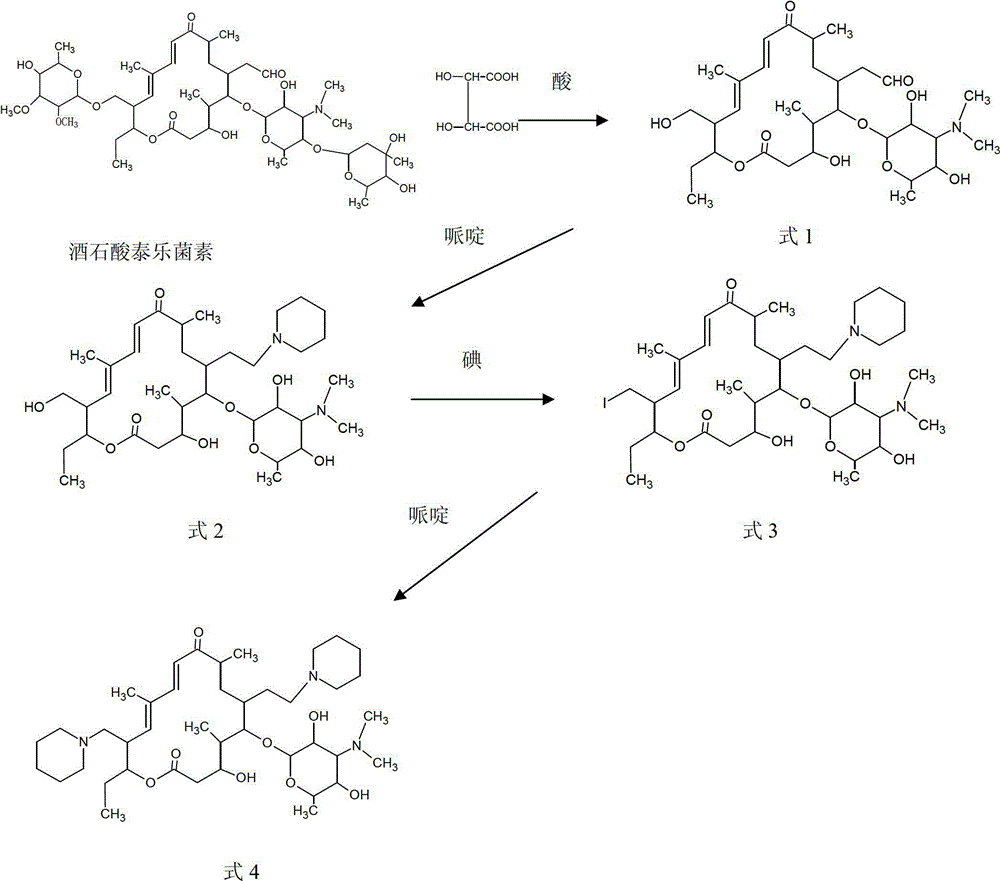
Process for preparing 20,23-bi-piperidyl-5-O-carbon mould amine glycosyl-tylosin lactone
A technology of mycaminosyl and tylonolactone, which is applied to the preparation of sugar derivatives, chemical instruments and methods, sugar derivatives, etc., can solve the problems of cumbersome reaction steps, high cost, and many by-products, and achieve purity High, easy industrial application, and simplified production process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
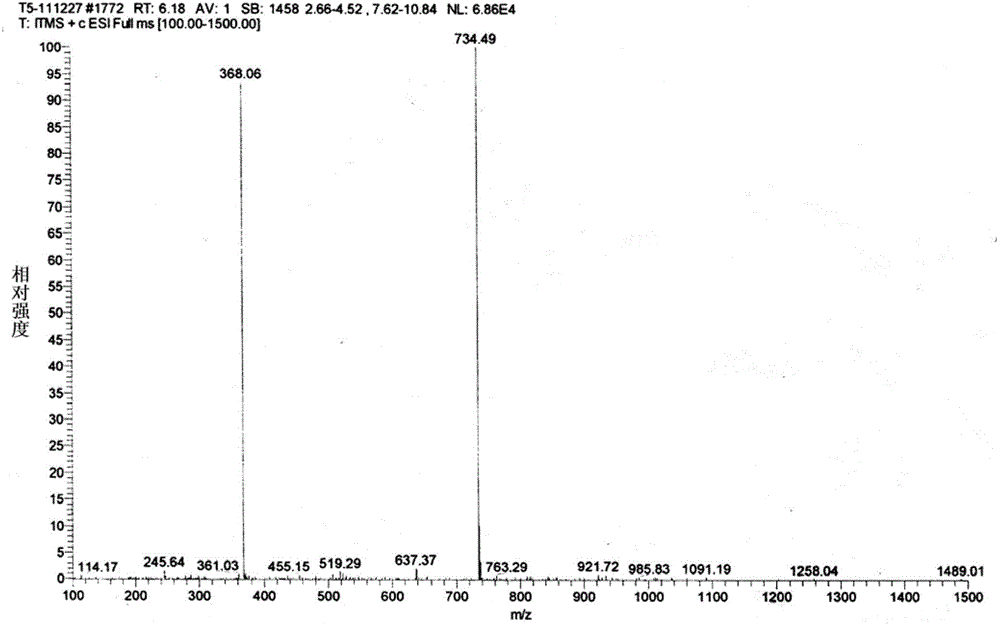
[0046] A preparation method of 20,23-dipiperidinyl-5-O-mycaminosyl-tylonolide, the steps are as follows:
[0047] (1) Dissolve 300kg of tylosin tartrate in 4500kg of purified water, then add 741.6kg of sulfuric acid with a mass percentage of 20% while keeping the temperature at 50°C, and hydrolyze for 8 hours. The peak of tylosin detected by HPLC completely disappears. Detected by HPLC, 150kg of 23-hydroxyl-5-O-mycaminosyl-tylonolide was obtained, with a molar yield of 82.8%;
[0048] (2) Add 755 kg of dichloromethane to the 150 kg reaction liquid containing 23-hydroxy-5-O-mycaminosyl-tylonolactone prepared in step (1), and adjust the pH with 5 mol / L sodium hydroxide solution When the value reaches 8, separate layers, take the organic layer, add 21.5kg of piperidine, 11.5kg of anhydrous formic acid, heat to 40°C, reflux for 5h, and detect 23-hydroxy-5-O-mycaminosyl-Taylor by HPLC The lactone content is less than 2g / L, and 149kg of 20-piperidinyl-23-hydroxy-5-O-mycaminosyl-tyl...
Embodiment 2
[0053] A preparation method of 20,23-dipiperidinyl-5-O-mycaminosyl-tylonolide, the steps are as follows:
[0054] (1) Dissolve 300kg of tylosin tartrate in 5400kg of purified water, then add 370.8kg of sulfuric acid with a mass percentage of 40% at a temperature of 80°C, and hydrolyze for 6 hours. The peak of tylosin detected by HPLC completely disappears. Detected by HPLC, 151 kg of 23-hydroxyl-5-O-mycaminosyl-tylonolide was obtained, with a molar yield of 83.4%;
[0055] (2) Add 755kg of dichloromethane to the 151kg reaction liquid containing 23-hydroxy-5-O-mycaminosyl-tylonolactone prepared in step (1), and adjust the pH with 5mol / L sodium hydroxide solution When the value reaches 10, separate layers, take the organic layer, add 25.8kg of piperidine, 13.8kg of anhydrous formic acid, heat to 40°C, reflux for 5.5h, and detect 23-hydroxy-5-O-mycaminosyl-Thai by HPLC The content of tylonolide is less than 2g / L, and 155kg of 20-piperidinyl-23-hydroxyl-5-O-mycaminosyl-tylonolide...
Embodiment 3
[0060] A preparation method of 20,23-dipiperidinyl-5-O-mycaminosyl-tylonolide, the steps are as follows:
[0061] (1) Dissolve 300kg of tylosin tartrate in 6000kg of purified water, then add 302kg of sulfuric acid with a mass percentage of 98% while keeping the temperature at 90°C, and hydrolyze for 6 hours. The peak of tylosin detected by HPLC disappears completely. Detected and obtained 130 kg of 23-hydroxy-5-O-mycaminosyl-tylonolide, with a molar yield of 71.8%;
[0062] (2) Add 755 kg of dichloromethane to the 130 kg reaction liquid containing 23-hydroxy-5-O-mycaminosyl-tylonolactone prepared in step (1), and adjust the pH with 5 mol / L sodium hydroxide solution When the value reaches 12, separate layers, take the organic layer, add 43kg of piperidine, 26kg of anhydrous formic acid, heat to 40°C, reflux for 6h, and detect the content of 23-hydroxy-5-O-mycaminosyl-tylonolide by HPLC Less than 2g / L, 138kg of 20-piperidinyl-23-hydroxyl-5-O-mycaminosyl-tylonolide was obtained;...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com