Preparation method of tylosin tartrate-doxycycline hydrochloride pharmaceutical composition
A technology of tylosin tartrate and doxycycline hydrochloride, applied in drug delivery, antibacterial drugs, pharmaceutical formulations, etc., can solve the problems of drug waste, affecting feed intake and drinking water of livestock and poultry, and poor palatability, etc. achieve good curative effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
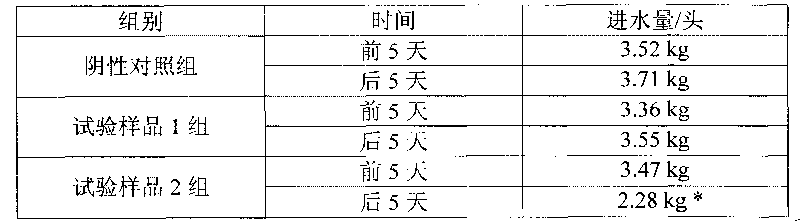
Image
Examples
Embodiment 1
[0022] The preparation of embodiment 1 tylosin tartrate microspheres
[0023] (1) Take 100 g of ethyl cellulose, add 1000 ml of cyclohexane, heat and stir to dissolve and make ethyl cellulose solution;
[0024] (2) Take 60 g of tylosin tartrate fine powder, disperse it uniformly in the above solution, heat to 80° C., stir for 30 min, and cool to room temperature to obtain a suspension of dispersed microspheres;
[0025] (3) The suspension of dispersed microspheres was centrifuged at 3000 rpm for 20 min, filtered and dried to obtain tylosin tartrate microspheres.
[0026] The tylosin tartrate microspheres were tested and found to have good roundness and good dispersibility in aqueous solution, with an average particle size of 38.5 μm, and 87% of the microspheres had a particle size distribution range of 60 to 180 μm. The encapsulation efficiency is 52.6%, and the drug loading is 28.4%.
Embodiment 2
[0027] The preparation of embodiment 2 tylosin tartrate microspheres
[0028] (1) Take polyethylene glycol-4000 140g, heat and stir with 2400ml ethanol to dissolve, prepare PEG solution;
[0029] (2) Heat and melt 40g of paraffin wax, add 60g of tylosin tartrate powder, stir quickly and evenly disperse in the above solution while hot, stir at 80°C for 30min, cool to room temperature to obtain a suspension;
[0030] (3) The suspension of dispersed microspheres was centrifuged at 3000 rpm for 20 min, filtered, washed with petroleum ether, and dried to obtain tylosin tartrate microspheres.
[0031] The tylosin tartrate microspheres were tested and found to have good roundness and good dispersibility in aqueous solution, with an average particle size of 22.4 μm, and microspheres with a particle size distribution range of 60 to 180 μm accounted for 76% of the total. The encapsulation efficiency is 71.2%, and the drug loading is 21.6%.
Embodiment 3
[0032] The preparation of embodiment 3 doxycycline hydrochloride microspheres
[0033] (1) Take polyethylene glycol-4000 40g, heat and stir with 600ml ethanol to dissolve, prepare PEG solution;
[0034] (2) Take 10 g of paraffin and heat to melt, add 35 g of doxycycline hydrochloride powder, stir quickly and evenly disperse in the above solution while hot, stir at 80°C for 30 min, cool to room temperature to obtain a suspension;
[0035] (3) The suspension of dispersed microspheres was centrifuged at 3000 rpm for 20 min, filtered, washed with petroleum ether, and dried to obtain doxycycline hydrochloride microspheres.
[0036] The doxycycline hydrochloride microspheres were tested and found to have good roundness and good dispersibility in aqueous solution, with an average particle size of 30.1 μm, and microspheres with a particle size distribution range of 60 to 180 μm accounted for 82% of the total. The encapsulation efficiency is 64.6%, and the drug loading is 33.2%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com