Acetylisovaleryl Tylosin Tartrate clathration enteric-coated preparation and preparing method thereof
A technology of tyvanectin and enteric-coated preparations, which is applied in the field of veterinary antibiotic preparations, can solve problems such as easy flying and falling palatability, decreased drug efficacy, and poor feed intake, so as to improve the therapeutic effect, save production costs, and ensure local effect of concentration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] A tyvanin tartrate inclusion enteric preparation, and its preparation method is as follows:
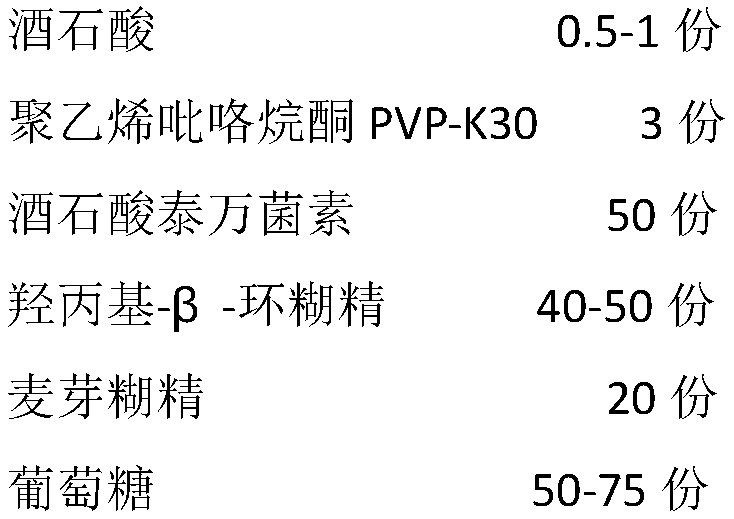
[0034] (1) Add 0.5 kg of tartaric acid and 3 kg of polyvinylpyrrolidone PVP-K30 to 50 kg of water, heat to 50°C to dissolve, and prepare solution A;
[0035] (2) Add 50 kg of tyvanin tartrate into solution A, keep the temperature at 50°C, and stir for 15 minutes to prepare a tyvanin tartrate solution;
[0036] (3) Dissolve 50kg of hydroxypropyl-β-cyclodextrin in 100kg of water to prepare solution B;
[0037] (4) Add the tyvans tartrate solution to solution B, stir and mix for 30 min, to obtain the tyvans tartrate inclusion compound solution.
[0038] (5) Add 20 kg of maltodextrin to the tyvanin tartrate inclusion compound solution in step (4), and stir to dissolve the drug-containing binder.
[0039] (6) Put 75kg glucose in the fluidized bed coating machine, the inlet air temperature is 80℃, the fan frequency is 35Hz, the spray frequency is 85Hz, and spray the drug-containing adhesive obt...
Embodiment 2
[0043] A tyvanin tartrate inclusion enteric preparation, and its preparation method is as follows:
[0044] (1) Add 0.5 kg of tartaric acid and 3 kg of polyvinylpyrrolidone PVP-K30 to 50 kg of water, heat to 50°C to dissolve, and prepare solution A;
[0045] (2) Add 50 kg of tyvanin tartrate into solution A, keep the temperature at 50°C, and stir for 15 minutes to prepare a tyvanin tartrate solution;
[0046] (3) Dissolve 40kg of hydroxypropyl-β-cyclodextrin in 100kg of water to prepare solution B;
[0047] (4) Add the tyvans tartrate solution to solution B, stir and mix for 30 min, to obtain the tyvans tartrate inclusion compound solution.
[0048] (5) Add 20 kg of maltodextrin to the tyvanin tartrate inclusion compound solution in step (4), and stir to dissolve the drug-containing binder.
[0049] (6) Put 50kg glucose in the fluidized bed coating machine, the inlet air temperature is 80℃, the fan frequency is 35Hz, the spray frequency is 85Hz, spray into the medicine-containing adhesiv...
Embodiment 3
[0054] A tyvanin tartrate inclusion enteric preparation, and its preparation method is as follows:
[0055] (1) Add 0.5 kg of tartaric acid and 3 kg of polyvinylpyrrolidone PVP-K30 to 50 kg of water, heat to 50°C to dissolve, and prepare solution A;
[0056] (2) Add 50 kg of tyvanin tartrate into solution A, keep the temperature at 50°C, and stir for 15 minutes to prepare a tyvanin tartrate solution;
[0057] (3) Dissolve 50kg of hydroxypropyl-β-cyclodextrin in 100kg of water to prepare solution B;
[0058] (4) Add the tyvans tartrate solution to solution B, stir and mix for 30 min, to obtain the tyvans tartrate inclusion compound solution.
[0059] (5) Add 20 kg of maltodextrin to the tyvanin tartrate inclusion compound solution in step (4), and stir to dissolve the drug-containing binder.
[0060] (6) Put 50kg glucose in the fluidized bed coating machine, the inlet air temperature is 80℃, the fan frequency is 35Hz, the spray frequency is 85Hz, spray into the medicine-containing adhesiv...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com