Long-acting compound ceftiofur suspension injection and its preparation method
A technology of ceftiofur and ceftiofur hydrochloride, which is applied in the field of veterinary drug preparations, can solve problems such as frequent drug use, high irritation at the injection site, and failure to achieve clinical effects, and achieve easy scale-up production, low tissue irritation, and The effect of increasing release amount and release speed
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
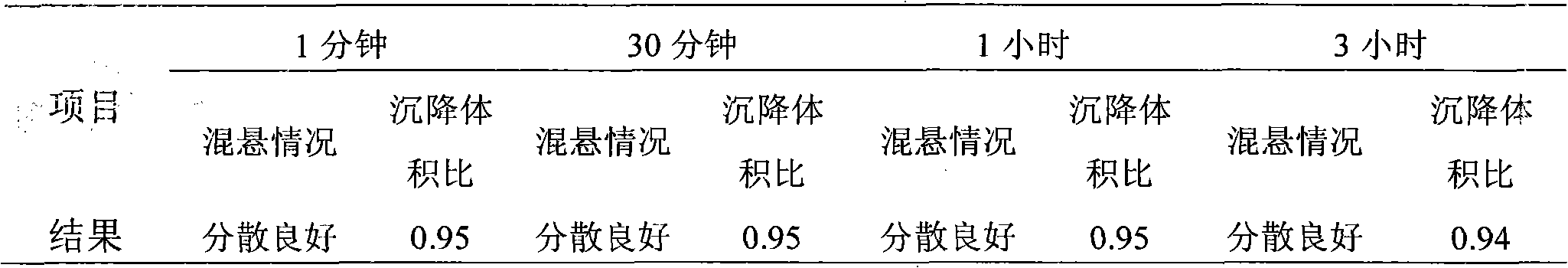
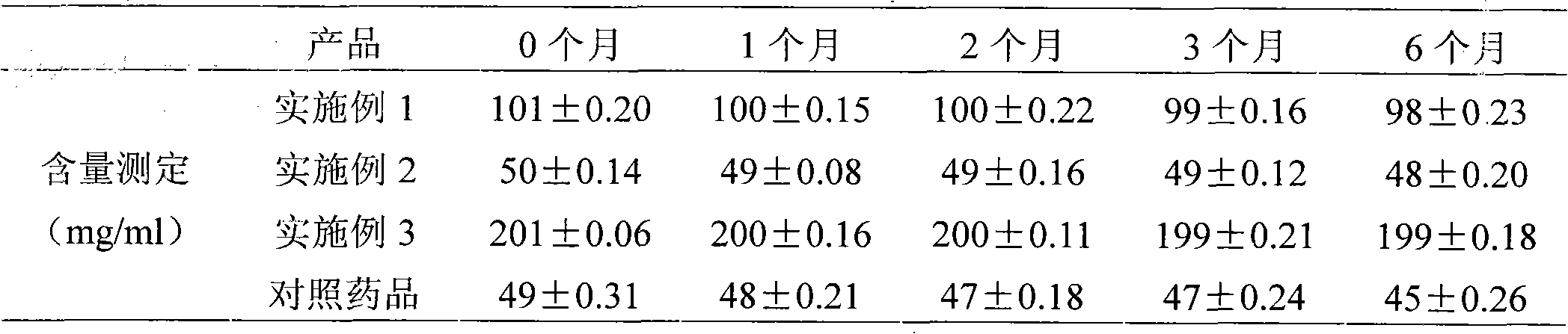
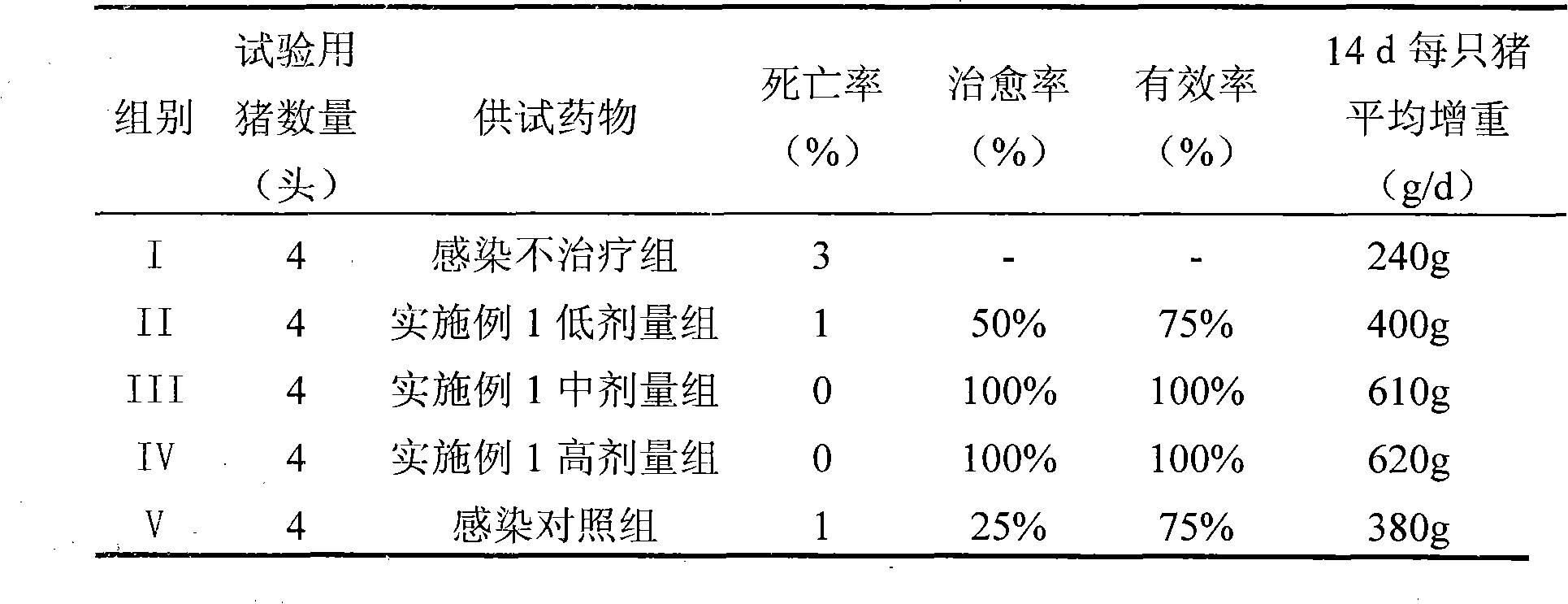
Image
Examples
Embodiment 1
[0025] Party:
[0026] Component Dosage
[0027] Ceftiofur Hydrochloride 1000g±5%
[0028] Tylosin Tartrate 1000g±5%
[0029] Aluminum stearate 200g±5%
[0030] Span-80 50g±5%
[0031] Propyl gallate 5g±5%
[0032] Add soybean oil for injection to 10L
[0033] Preparation:
[0034] A. Measure each component according to the prescription and set aside.
[0035] B. Mix soybean oil for injection, aluminum stearate and propyl gallate, add to a pressure cooker or an autoclave cabinet and heat to 120-125°C for 2 hours to form a gel.
[0036] C. Under stirring, cool the gel to below 40°C, prepare oil gel, and set aside.
[0037] D. Take the prescribed amount of ceftiofur hydrochloride and tylosin tartrate, add an appropriate amount of oil gel, and stir in a high-speed mixer.
[0038] F. Transfer the above-mentioned stirred liquid to a quantitative container, add the prescribed amount of Span-80 into the container, add the remaining oil glue to 10L, and stir while adding.
...
Embodiment 2
[0041] Party:
[0042] Component Dosage
[0043] Ceftiofur Hydrochloride 500g±5%
[0044] Tylosin Tartrate 500g±5%
[0045] Polyvinylpyrrolidone 100g±5%
[0046] Tween-80 100g±5%
[0047] Vitamin C Palmitate 2g±5%
[0048] Add tea oil for injection to 10L
[0049] Preparation:
[0050] A. Measure each component according to the prescription and set aside.
[0051] B. Mix camellia oil for injection, polyvinylpyrrolidone and vitamin C palmitate, add to a pressure cooker or an autoclave cabinet and heat to 120-125°C for 2 hours to make a gel.
[0052] C. Under stirring, cool the gel to below 40°C, prepare oil gel, and set aside.
[0053] D. Take the prescribed amount of ceftiofur hydrochloride and tylosin tartrate, add an appropriate amount of oil gel, and stir in a high-speed mixer.
[0054] F. Transfer the above-mentioned stirred liquid to a quantitative container, add the prescribed amount of Tween-80 into the container, add the remaining oil gel to 10L, and stir whil...
Embodiment 3
[0057] Party:
[0058] Component Dosage
[0059] Ceftiofur Hydrochloride 2000g±5%
[0060] Tylosin Tartrate 2000g±5%
[0061] Aluminum stearate 200g±5%
[0062] Soy lecithin 10g±5%
[0063] Span-80 200g±5%
[0064] tert-butyl p-hydroxyanisole 20g±5%
[0065] Dibutyl hydroxytoluene 20g±5%
[0066] Refined corn oil to 10L
[0067] Preparation:
[0068] A. Measure each component according to the prescription and set aside.
[0069] B. Mix refined corn oil, aluminum stearate, tert-butyl p-hydroxyanisole and dibutyl hydroxytoluene, add to a pressure cooker or autoclave cabinet and heat to 120-125°C for 2 hours to form a gel .
[0070] C. Under stirring, cool the gel to below 40°C, prepare oil gel, and set aside.
[0071] D. Take the prescribed amount of ceftiofur hydrochloride and tylosin tartrate, add an appropriate amount of oil gel, and stir in a high-speed mixer.
[0072] F. Transfer the above-mentioned stirred liquid to a quantitative container, add the prescribed am...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com