A kind of preparation method of lidocaine hydrochloride
A technology of lidocaine hydrochloride and hydrochloric acid, which is applied to the preparation of carboxylic acid amides, organic compounds, amino compounds, etc., to achieve the effects of simple synthesis process, high economy and environmental protection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
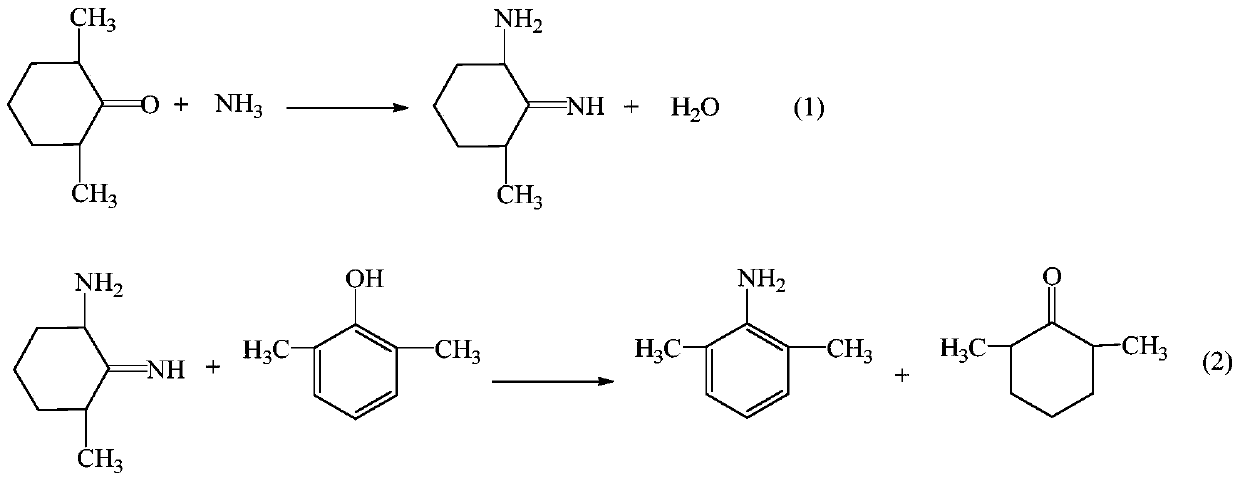
[0026] (1) Preparation of intermediate 2,6-dimethylaniline
[0027] Add 24.43g (0.2mol) of 2,6-xylenol, 63.1g (0.6mol) of ammonia water, 1.06g of 5% Pd / C, 2.52g (0.02 mol), react at 185°C for 6h, cool down to room temperature, filter, recover Pd / C and use it mechanically; distill the filtrate under reduced pressure, recover 2,6-dimethylcyclohexanone and use it mechanically next time, add water to wash the residue once, let stand and separate , the oil layer is 23.35 g of the intermediate 2,6-dimethylaniline, the HPLC purity is 98.32%, and the yield is 94.73%.
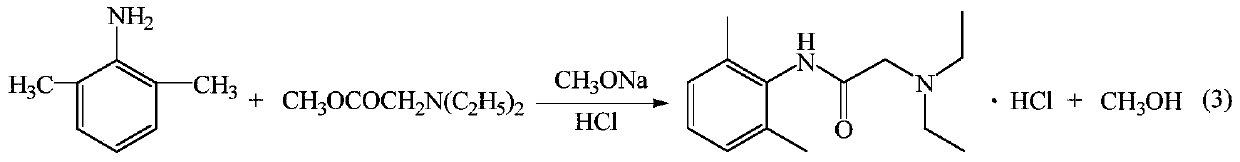
[0028] (2) preparation of lidocaine hydrochloride
[0029] Add 15.12 g (0.28 mol) of sodium methoxide, 24.24 g (0.2 mol) of intermediate 2,6-dimethylaniline and 31.9 g (0.22 mol), heated to 95°C, and distilled off the methanol generated during the reaction until no methanol was distilled out, then continued the reaction for 30 minutes, cooled to room temperature, added dichloroethane to dissolve, washed twice with wat...
Embodiment 2-3
[0031] Embodiment 2-3 Preparation of intermediate 2,6-dimethylaniline
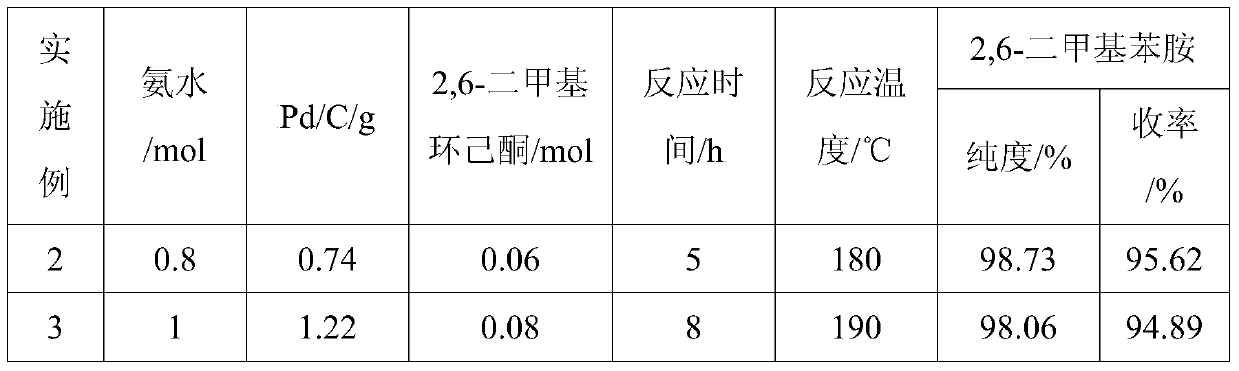
[0032] Using the same operating method as in Example 1 (1), the same amount of 2,6-xylenol, the difference is the amount of ammonia, Pd / C and 2,6-dimethylcyclohexanone, the experimental results obtained are shown in the table 1 shows:
[0033] Table 1:
[0034]
Embodiment 4
[0035] The preparation of embodiment 4 lidocaine hydrochloride
[0036] Add 12.96 g (0.24 mol) of sodium methoxide, 24.24 g (0.2 mol) of intermediate 2,6-dimethylaniline and 34.8 g (0.24 mol), heated to 90°C, and distilled off the methanol generated during the reaction until no methanol was distilled out, then continued the reaction for 30 minutes, cooled to room temperature, added dichloroethane to dissolve, washed twice with water, and stood to separate layers. The organic layer is the dichloroethane solution of lidocaine base.
[0037] In the ethylene dichloride solution of above-mentioned lidocaine base, add hydrochloric acid 2.42g, regulate PH with hydrogen chloride then to be 4, add gac and reflux 30min, filter, filtrate is concentrated, crystallization by cooling, dry to obtain lidocaine hydrochloride 48g, HPLC purity is 99.32%, the yield is 87.96%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com