Anti-rheumatoid arthritis drug gel containing paclitaxel liposome and preparation method of gel
A rheumatoid and paclitaxel technology, applied in anti-inflammatory agents, drug combinations, pharmaceutical formulations, etc., can solve problems such as easy aggregation, poor stability of liposome properties, and difficulty in long-term storage, so as to improve bioavailability and relieve pain Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
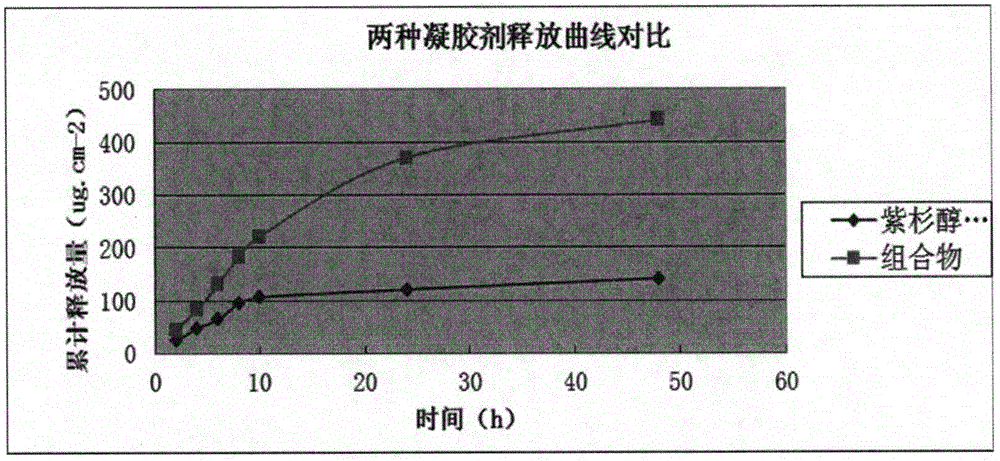
Image
Examples
Embodiment 1
[0027] Example 1: Preparation of paclitaxel liposomes:
[0028] prescription:
[0029] Process: Dissolve 0.15g paclitaxel, 1.8g soy lecithin, 0.8g cholesterol, and vitamin E into chloroform according to a certain proportion, put it in a pear-shaped bottle, slowly rotate and evaporate under reduced pressure on a rotary evaporator at 45℃, and pass in Nitrogen removes the organic solvent, so that the lipid film is evenly distributed on the flask. Mix 20 mL of the sucrose solution with the membrane for hydration for 30 minutes, and ultrasonically vibrate to obtain a white suspension, which is sheared 4 times with a high-pressure micro-jet nano-disperser with a power of 9 Kpsi to obtain a paclitaxel nano-liposome solution. Pre-freeze for 5h and freeze-dry.
Embodiment 2
[0030] Example 2: Preparation of paclitaxel liposomes:
[0031] prescription:
[0032] Process: Dissolve 0.1g of paclitaxel, 5.7g of soy lecithin, 0.5g of cholesterol, and appropriate amount of vitamin E in chloroform according to a certain ratio, place in a pear-shaped bottle, slowly rotate and evaporate under reduced pressure on a rotary evaporator at 45°C, pass in Nitrogen removes the organic solvent, so that the lipid film is evenly distributed on the flask. Mix 20 mL of the sucrose solution with the membrane for hydration for 30 minutes, and ultrasonically vibrate to obtain a white suspension, which is sheared 4 times with a high-pressure micro-jet nano-disperser with a power of 9 Kpsi to obtain a paclitaxel nano-liposome solution. Pre-freeze for 5h and freeze-dry.
Embodiment 3
[0033] Example 3: Preparation of paclitaxel liposomes:
[0034] prescription:
[0035] Process: Dissolve 0.06g of paclitaxel, 4.5g of soybean lecithin, 0.5g of cholesterol, and appropriate amount of vitamin E in chloroform according to a certain ratio, place in a pear-shaped bottle, slowly rotate and evaporate under reduced pressure on a rotary evaporator at 45℃, and pass in Nitrogen removes the organic solvent, so that the lipid film is evenly distributed on the flask. Mix 20 mL of the sucrose solution with the membrane for hydration for 30 minutes, and ultrasonically vibrate to obtain a white suspension, which is sheared 4 times with a high-pressure micro-jet nano-disperser with a power of 9 Kpsi to obtain a paclitaxel nano-liposome solution. Pre-freeze for 5h and freeze-dry.
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com