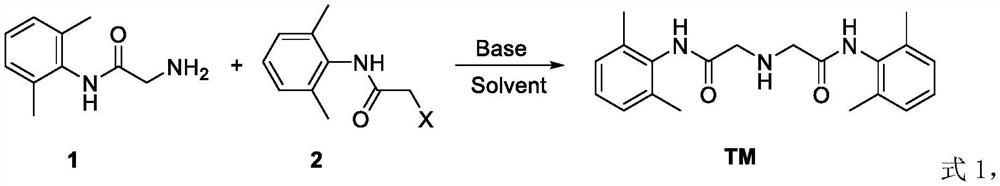
Preparation method of lidocaine hydrochloride impurity E
A technology for lidocaine hydrochloride and impurities, which is applied in the field of compound synthesis technology, can solve the problem of not synthesizing lidocaine hydrochloride impurity E and the like, and achieves the effects of high purity, safe reaction and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
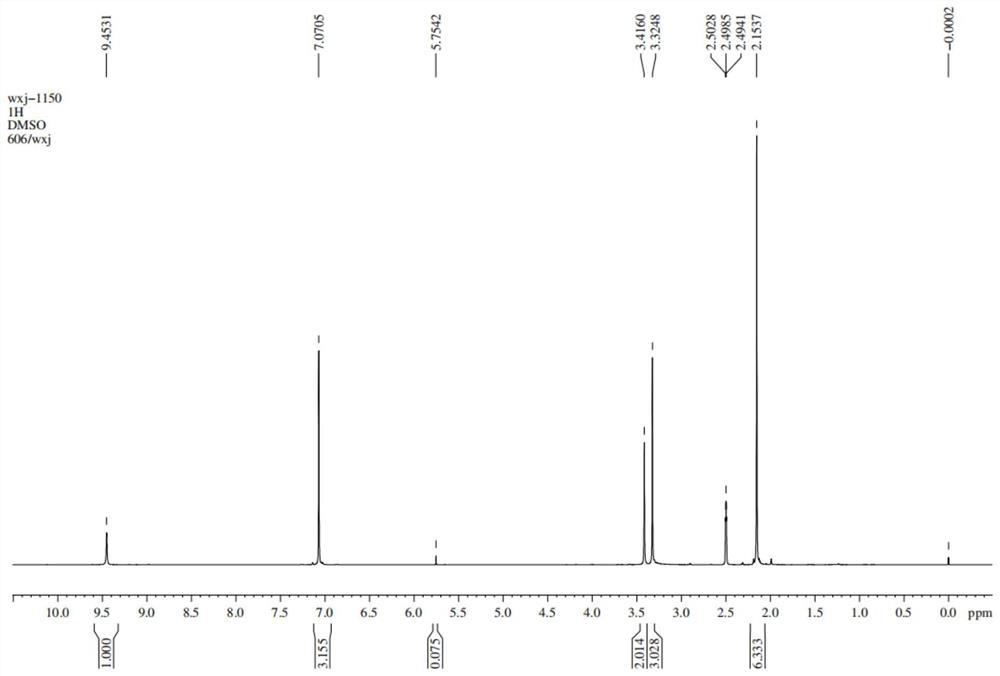
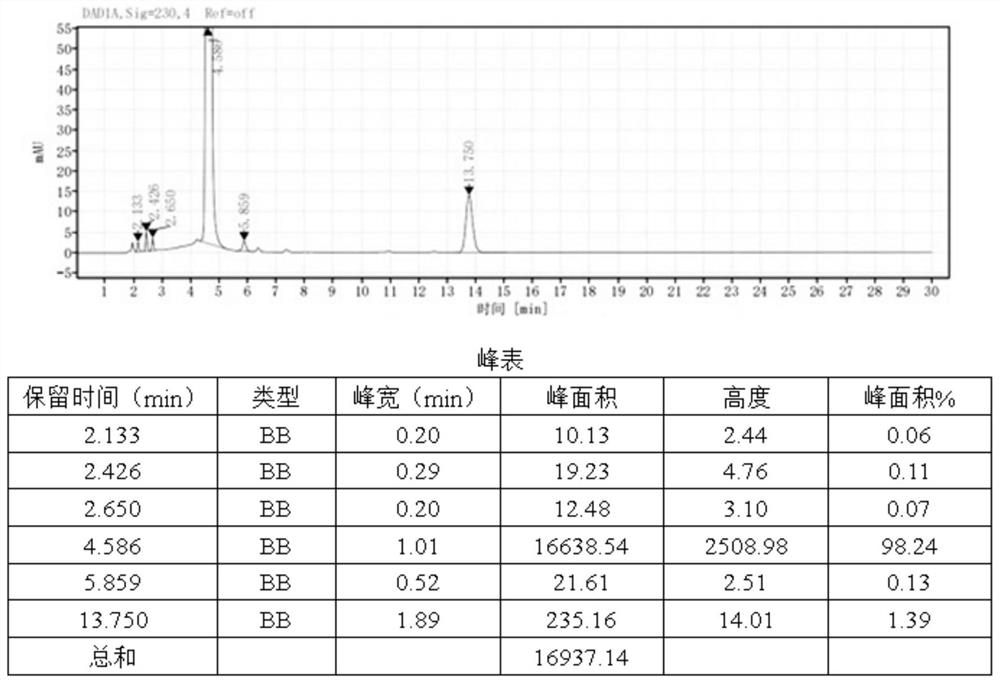
[0025] The preparation method of the lidocaine hydrochloride impurity E of the present embodiment comprises the following steps: add 20g of compound 1, i.e. 4-morpholine-3-aniline, and 35ml of solvent DMF to a reactor with a thermometer and a stirring paddle , and then add 21.1 g of compound 2a, namely 2-chloro-N-(2,6-xylyl) acetamide, under stirring conditions at room temperature, and after stirring for 30 min, add 12.3 g of diethylamine, and react for 40 min at 25°C , raised the temperature to 60°C for 2 hours, then raised the temperature to 90°C for 3 hours, added water and ethyl acetate, washed the organic layer with saturated brine, dried over anhydrous sodium sulfate, filtered, and removed the solvent in vacuo to obtain 31.2 g of lidocaine hydrochloride Impurity E has a purity of 98.2% and a yield of 89.2%. The reaction formula is shown in formula 2.
[0026]
Embodiment 2
[0028] The preparation method of the lidocaine hydrochloride impurity E of the present embodiment comprises the steps of: adding 20 g of compound 1, i.e. 4-morpholine-3-aniline, and 35 ml of solvent two to a reactor with a thermometer and a stirring paddle Chloromethane, then add 32.6g of compound 2b, namely 2-bromo-N-(2,6-xylyl)acetamide, under stirring at room temperature, and after stirring for 20min, add 28.9g of N,N-diisopropyl Ethylamine, react at 30°C for 50min, raise the temperature to 45°C for 3h, then raise the temperature to 100°C for 2h, add water and ethyl acetate, wash the organic layer with saturated saline, dry over anhydrous sodium sulfate, filter, and remove the solvent in vacuo , to obtain 34.1 g of lidocaine hydrochloride impurity E, with a purity of 98.6% and a yield of 88.2%. The reaction formula is shown in formula 3.
[0029]
Embodiment 3
[0031] The preparation method of the lidocaine hydrochloride impurity E of the present embodiment comprises the following steps: add 20g of compound 1, i.e. 4-morpholine-3-aniline, and 35ml of solvent DMSO to a reactor with a thermometer and a stirring paddle , and then added 32.4 g of compound 2c, namely 2-methoxy-N-(2,6-xylyl) acetamide, under stirring conditions at room temperature, and after stirring for 40 min, added 34.0 g of triethylamine, at 30 ° C React for 30 minutes, raise the temperature to 45°C for 1.5h, then raise the temperature to 80°C for 4h, add water and ethyl acetate, wash the organic layer with saturated brine, dry over anhydrous sodium sulfate, filter, and remove the solvent in vacuo to obtain 35.0g of hydrochloric acid Lidocaine impurity E has a purity of 98.0% and a yield of 90.6%. The reaction formula is shown in formula 4.
[0032]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com