Method for detecting content of lidocaine hydrochloride in medical cross-linked sodium hyaluronate gel
A technology of cross-linking hyaluronic acid and lidocaine hydrochloride, which is applied in the direction of measuring devices, instruments, scientific instruments, etc., can solve the problems that affect the accuracy and repeatability of test results, and cannot filter membranes of lidocaine hydrochloride, etc., to achieve repeatability High, easy to operate, good detection accuracy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
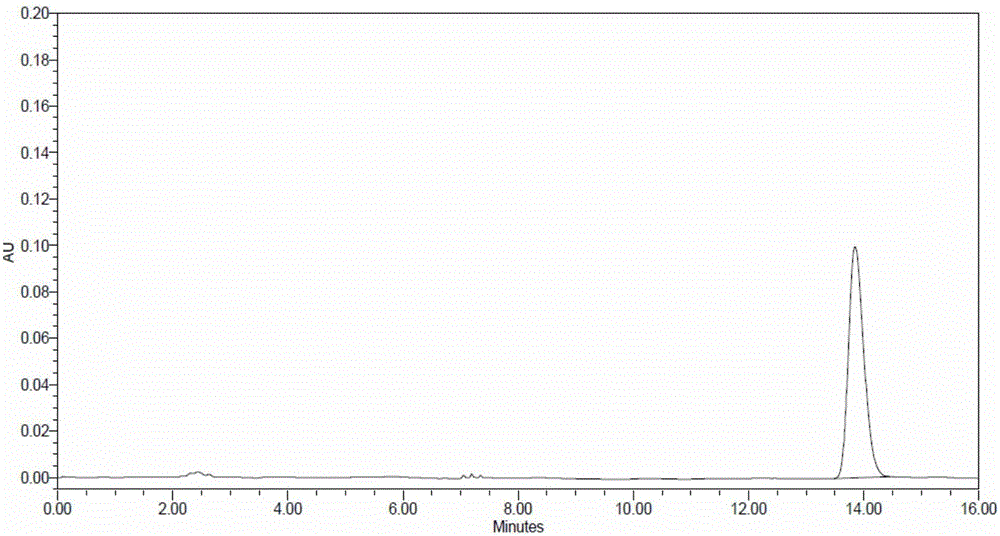
[0032] Mobile phase preparation: 1.3ml of 1mol / L sodium dihydrogen phosphate solution and 32.5ml of 0.5mol / L disodium hydrogen phosphate solution, diluted with water to 1000ml, phosphate solution: acetonitrile=500:500, adjust the pH to 8.0
[0033] Reference substance solution preparation: take lidocaine reference substance 15.6mg, dilute to 0.312mg / ml with mobile phase, set aside.
[0034] Preparation of the test solution: take 1.1014g of the product, use 4.0ml of sodium hyaluronate enzyme solution with a concentration of 60U / ml, keep the temperature in a water bath at 37°C for 24 hours, and dilute to 10ml with mobile phase after dissolving, filter with a 0.45μm membrane Filter and set aside.
[0035] Detection:
[0036] Instrument: high performance liquid chromatography, octadecylsilane bonded silica gel column
[0037] Column temperature: 25°C, flow rate: 1ml / min
[0038] Precisely take 20 μL each of the test solution and the reference solution and inject it into the liq...
Embodiment 2
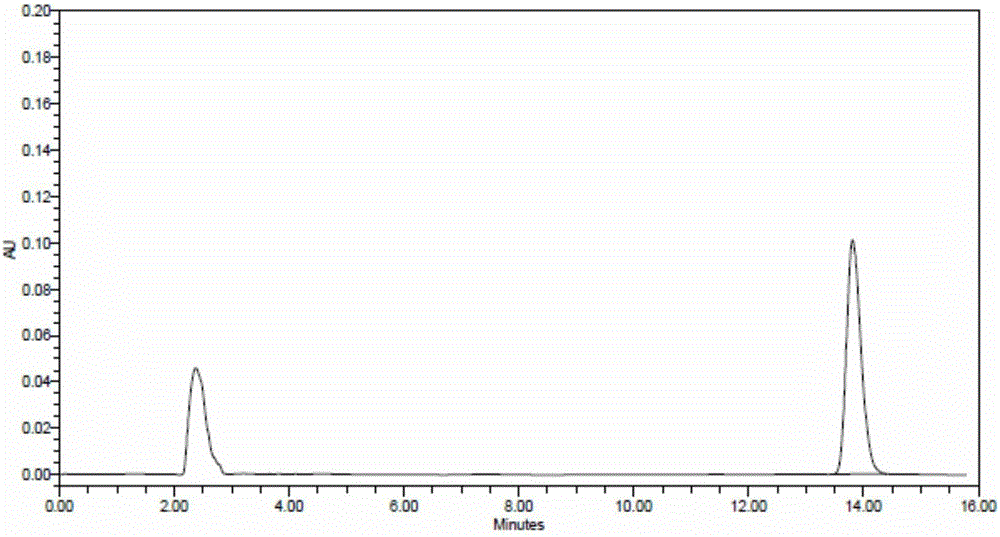
[0042] Mobile phase preparation: 1.3ml of 1mol / L sodium dihydrogen phosphate solution and 32.5ml of 0.5mol / L disodium hydrogen phosphate solution, diluted with water to 1000ml, phosphate solution: acetonitrile=500:500, adjust the pH to 8.0
[0043] Reference substance solution preparation: take lidocaine reference substance 15.1 mg, dilute to about 0.302 mg / ml with mobile phase, and set aside.
[0044] Preparation of the test solution: take 1.0689g of the product, use 4.0ml of sodium hyaluronate enzyme solution with a concentration of 50U / ml, keep the temperature in a water bath at 37°C for 24 hours, and dilute to 10ml with mobile phase after dissolving, filter with a 0.45μm membrane Filter and set aside.
[0045] Detection:
[0046] Instrument: high performance liquid chromatography, octadecylsilane bonded silica gel column
[0047] Column temperature: 25°C, flow rate: 1ml / min
[0048] Precisely take 20 μL each of the test solution and the reference solution and inject it ...
Embodiment 3
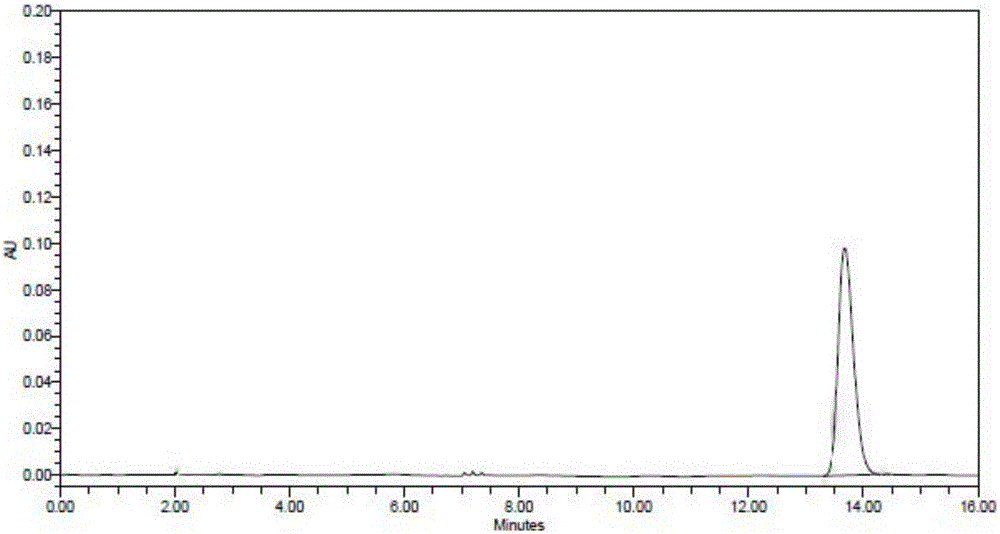
[0052] Mobile phase preparation: 1.3ml of 1mol / L sodium dihydrogen phosphate solution and 32.5ml of 0.5mol / L disodium hydrogen phosphate solution, diluted with water to 1000ml, phosphate solution: acetonitrile=500:500, adjust the pH to 8.0
[0053] Reference substance solution preparation: take lidocaine reference substance 16.6mg, dilute to about 0.332mg / ml with mobile phase, set aside.
[0054] Preparation of the test solution: take 1.1826g of the product, use 4.0ml of sodium hyaluronate enzyme solution with a concentration of 100U / ml, keep the temperature in a water bath at 37°C for 16 hours, dilute to 10ml with mobile phase after dissolving, and use a 0.45μm filter membrane Filter and set aside.
[0055] Detection:
[0056] Instrument: high performance liquid chromatography, octadecylsilane bonded silica gel column
[0057] Column temperature: 25°C, flow rate: 1ml / min
[0058] Precisely take 20 μL each of the test solution and the reference solution and inject it into t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com