Lidocaine hydrochloride polymeric liposome for topical anesthesia and preparation method
A technology of lidocaine hydrochloride and surface anesthesia, which is applied in the field of biomedicine, can solve the problems of poor product stability, low drug encapsulation rate, and difficult surface modification, etc. The effect of high encapsulation rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] The process of preparing lidocaine hydrochloride polymer liposomes by reverse-phase evaporation method is as follows:
[0039] (1) Accurately weigh 16 mg of OQLCS and 8 mg of cholesterol into an eggplant-shaped bottle, and dissolve in 4 ml of dichloromethane.
[0040] (2) Accurately weigh 40 mg of lidocaine hydrochloride, add it to an eggplant-shaped bottle, add 2 ml of deionized water, and dissolve completely.
[0041] (3) Add (2) to (1), and then ultrasonically disperse it with a probe-type ultrasonic generator at a power of 150 W until a translucent emulsion is formed. The above emulsion was rotary evaporated on a rotary evaporator at a rotation speed of 50 r / min at 40° C., and at the same time, a nitrogen flow was passed into the rotary evaporator for protection.
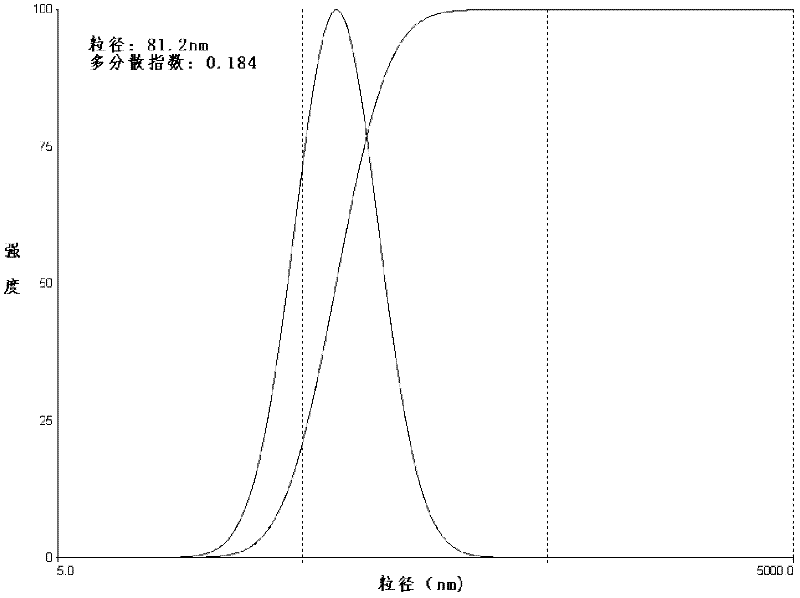
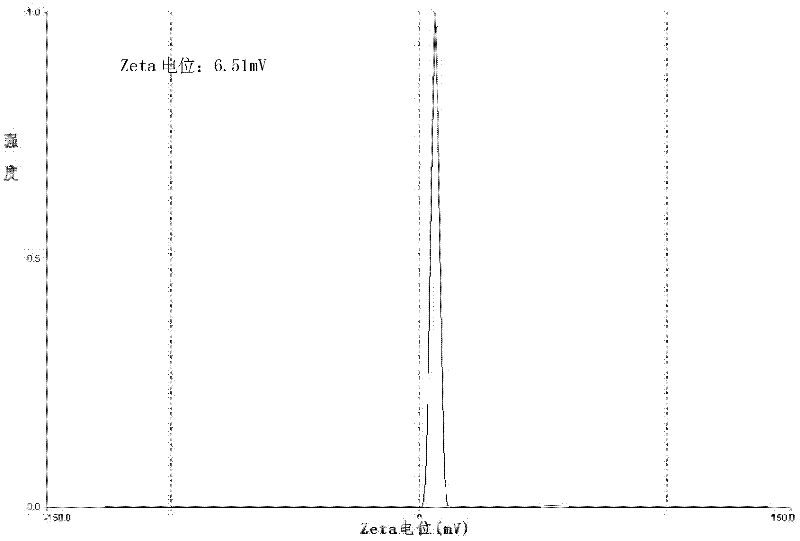
[0042] (4) After the organic solvent in the eggplant-shaped bottle is completely volatilized, the product is obtained by filtering through a 0.2 μm sterile filter membrane. The prepared lidocaine hydroc...
Embodiment 2
[0045] The process of preparing lidocaine hydrochloride polymer liposomes by reverse-phase evaporation method is as follows:
[0046] (1) Accurately weigh 48mg of OQLCS and 24mg of cholesterol into an eggplant-shaped bottle, and dissolve in 4ml of dichloromethane.
[0047] (2) Accurately weigh 80 mg of lidocaine hydrochloride, add it to an eggplant-shaped bottle, add 4 ml of deionized water, and dissolve completely.
[0048] (3) Add (2) to (1), and then ultrasonically disperse it with a probe-type ultrasonic generator at a power of 175W until a translucent emulsion is formed. The above emulsion was rotary evaporated on a rotary evaporator at a rotation speed of 50 r / min at 40° C., and at the same time, a nitrogen flow was passed into the rotary evaporator for protection.
[0049] (4) After the organic solvent in the eggplant-shaped bottle is completely volatilized, it is finally filtered through a 0.2 μm sterile filter membrane to obtain the product. The properties of the pr...
Embodiment 3
[0054] The process of preparing lidocaine hydrochloride polymer liposomes by reverse-phase evaporation method is as follows:
[0055] (1) Accurately weigh 160mg of OQLCS and 80mg of cholesterol into an eggplant-shaped bottle, and dissolve in 4ml of dichloromethane.
[0056] (2) Accurately weigh 80 mg of lidocaine hydrochloride, add it to an eggplant-shaped bottle, add 4 ml of deionized water, and dissolve completely.
[0057] (3) Add (2) to (1), and then ultrasonically disperse it with a probe-type ultrasonic generator at a power of 200 W until a translucent emulsion is formed. The above emulsion was rotary evaporated on a rotary evaporator at a rotation speed of 50 r / min at 40° C., and at the same time, a nitrogen flow was passed into the rotary evaporator for protection.
[0058] (4) After the organic solvent in the eggplant-shaped bottle is completely volatilized, the product is obtained by filtering through a 0.4 μm sterile filter membrane. The properties of the prepared...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Electric potential | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com