Lidocaine hydrochloride injection and preparation method thereof
A technology of lidocaine hydrochloride and lidocaine hydrochloride amount is applied in the directions of pharmaceutical formulations, medical preparations containing active ingredients, drug delivery, etc., which can solve the problem of insignificant anesthesia effect, poor solution stability, and increased risk of clinical medication. To reduce clinical adverse reactions, ensure safety and effectiveness, and eradicate clinical risks
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
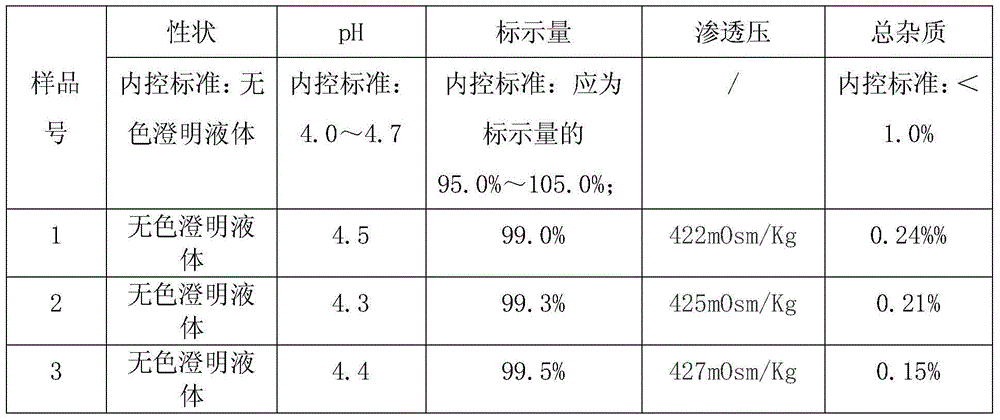
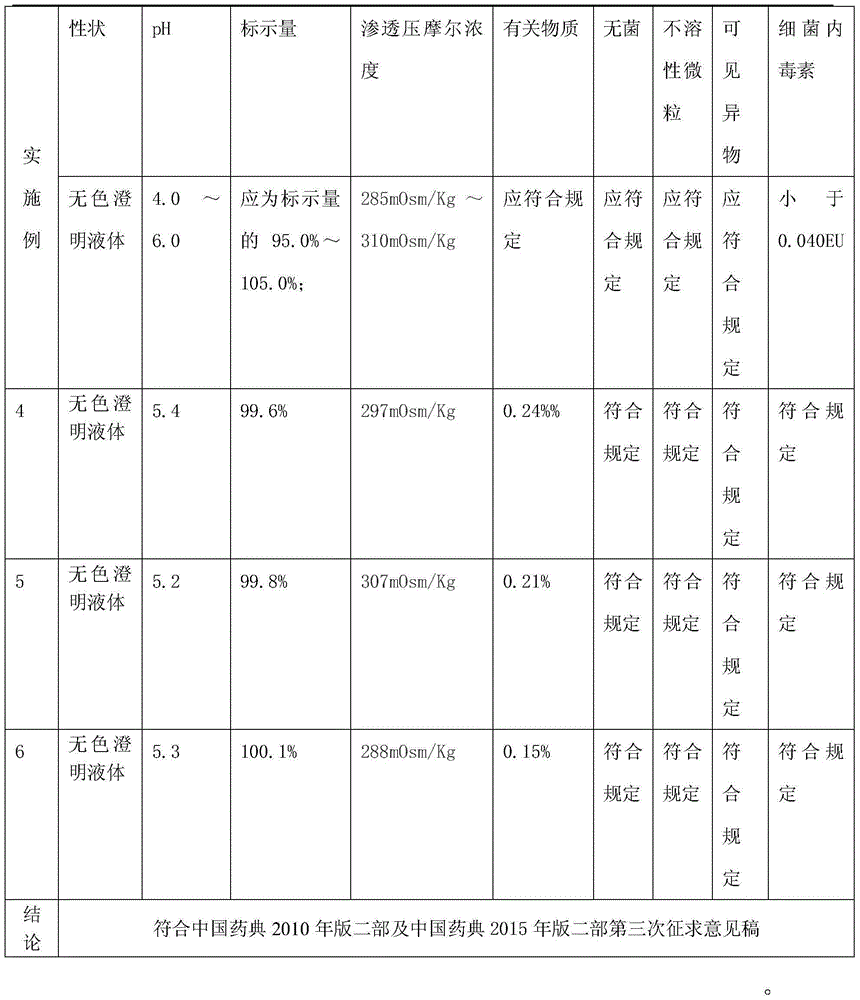
Examples
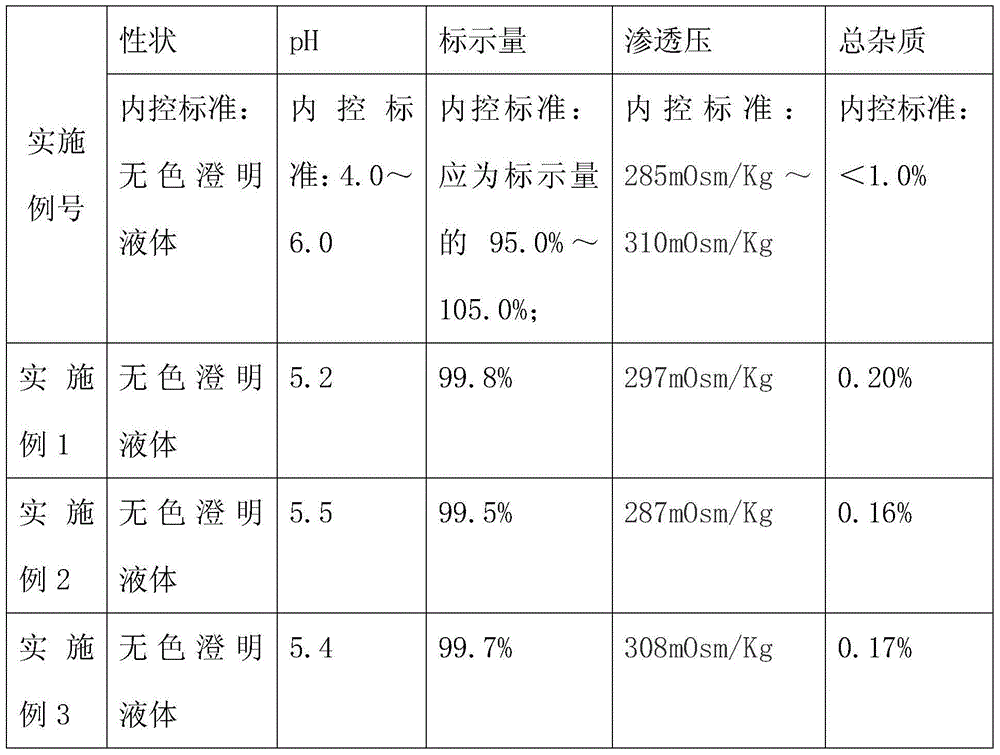
example 1
[0047] Example 1 prepares lidocaine hydrochloride injection:
[0048] serial number
Element
1000ml recipe volume
1
21.565g
2
4.9g
3
Add to 1000ml
[0049] (1), preparation: take by weighing sodium chloride, lidocaine hydrochloride, medicinal charcoal according to formula quantity, for subsequent use.
[0050] (2) Washing and drying the bottle: use an ultrasonic washing machine to clean the ampoule.
[0051] (3), dosing:
[0052] (3.1), put the water for injection of 70% formula quantity into the liquid mixing tank, add the sodium chloride of formula quantity, turn on the mixer and stir for 5 minutes.
[0053] (3.2), add the lidocaine hydrochloride of formula quantity in the liquid mixing tank, stir for 5 minutes.
[0054] (3.3), add medicinal charcoal according to the formula, and stir for 5 minutes.
[0055] (3.4), add water for injection, turn on...
example 2
[0063] Example 2 prepares lidocaine hydrochloride injection:
[0064] serial number
Element
1000ml recipe volume
1
Lidocaine Hydrochloride
21.565g
2
Sodium chloride
4.4g
3
Add to 1000ml
[0065] Remarks: lidocaine hydrochloride is fed according to the content and water content of the dry product.
[0066] (1), preparation: take by weighing sodium chloride, lidocaine hydrochloride, medicinal charcoal according to formula quantity, for subsequent use.
[0067](2) Washing and drying the bottle: use an ultrasonic washing machine to clean the ampoule.
[0068] (3), dosing:
[0069] (3.1), put the water for injection of 70% formula quantity into the liquid mixing tank, add the sodium chloride of formula quantity, turn on the mixer and stir for 5 minutes.
[0070] (3.2), add the lidocaine hydrochloride of formula quantity in the liquid mixing tank, stir for 5 minutes.
[0071] (3.3), add medici...
example 3
[0080] Example 3 prepares lidocaine hydrochloride injection:
[0081] serial number
Element
1000ml recipe volume
1
Lidocaine Hydrochloride
21.565g
2
Sodium chloride
5.3g
3
Add to 1000ml
[0082] Remarks: Lidoca hydrochloride is divided into dry and pure according to the content and water content.
[0083] (1), preparation: take by weighing sodium chloride, lidocaine hydrochloride, medicinal charcoal according to formula quantity, for subsequent use.
[0084] (2) Washing and drying the bottle: use an ultrasonic washing machine to clean the ampoule.
[0085] (3), dosing:
[0086] (3.1), put the water for injection of 70% formula quantity into the liquid mixing tank, add the sodium chloride of formula quantity, turn on the mixer and stir for 5 minutes.
[0087] (3.2), add the lidocaine hydrochloride of formula quantity in the liquid mixing tank, stir for 5 minutes.
[0088] (3.3), add medic...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com