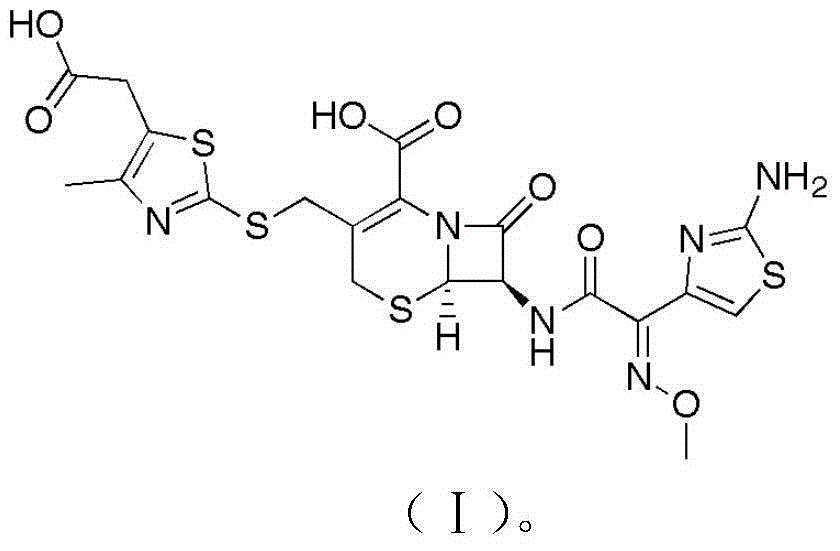
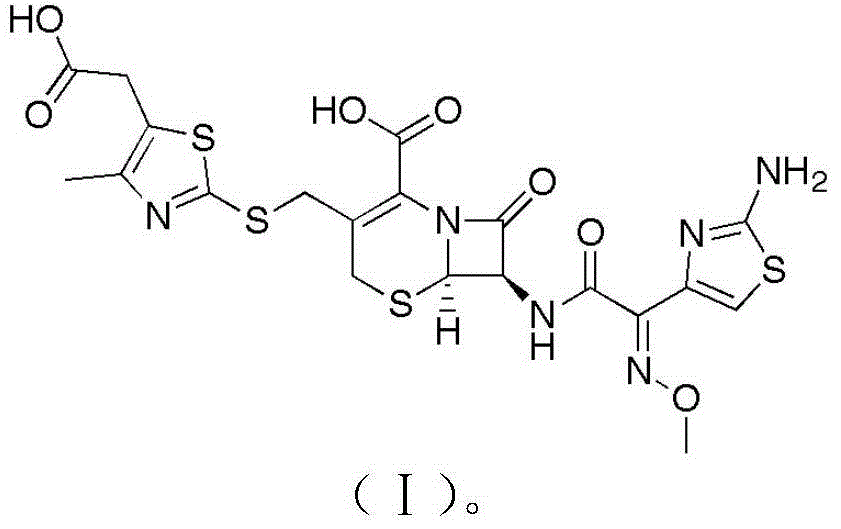
Children cefodizime sodium compound entity and preparation thereof
A technology of cefodizime sodium and compound, which is applied in the field of cefodizime sodium compound entity and its preparation for children, can solve the problems of unsatisfactory material index, increase the risk of preparation use, etc., so as to improve the safety of the drug, improve the Product stability and the effect of improving extraction efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] (1) Weigh 100 g of the crude product of cefodizime sodium, add 1000 ml of water, heat up to 30° C. to completely dissolve, add 10 g of activated carbon, stir for decolorization, and filter;
[0024] (2) Add 10ml of ethyl acetate under stirring, transfer to a 1000ml pressure-resistant container, make sure it is full and air bubbles are removed, seal the container, oscillate, and freeze at -18°C for 8 hours before taking it out;
[0025] (3) Remove the organic phase. After the ice melts, control the temperature at 10-15°C, slowly add 3000ml of ethanol dropwise for about 1 hour under the protection of nitrogen, cool down to 0-5°C and continue growing crystals for 1h, filter with suction, and wash with ethanol for 40 ℃ vacuum drying.
Embodiment 2
[0027] (1) Weigh 200 g of the crude product of cefodizime sodium, add 1000 ml of water, heat up to 30° C. until completely dissolved, add 10 g of activated carbon, stir for decolorization, and filter;
[0028] (2) Add 10ml of chloroform under stirring, transfer to a 1000ml pressure-resistant container, make sure it is full and the air bubbles are removed, seal the container, oscillate, and freeze at -18°C for 3 hours before taking it out;
[0029] (3) Remove the organic phase. After the ice melts, control the temperature at 10-15°C, slowly add 3000ml of ethanol dropwise for about 1 hour under the protection of nitrogen, cool down to 0-5°C and continue growing crystals for 1h, filter with suction, and wash with ethanol for 40 ℃ vacuum drying.
Embodiment 3
[0031] (1) Weigh 100 g of the crude product of cefodizime sodium, add 1000 ml of water, heat up to 30° C. to completely dissolve, add 10 g of activated carbon, stir for decolorization, and filter;
[0032] (2) Add 7ml of ethyl acetate under stirring, transfer to a 1000ml pressure-resistant container, make sure it is full and air bubbles are removed, seal the container, oscillate, freeze at -18°C for 8 hours, and then take it out;
[0033] (3) Remove the organic phase. After the ice melts, control the temperature at 10-15°C, slowly add 3000ml of ethanol dropwise for about 1 hour under the protection of nitrogen, cool down to 0-5°C and continue growing crystals for 1h, filter with suction, and wash with ethanol for 40 ℃ vacuum drying.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com