Preparation method of cefodizime sodium for injection
A technology for cefodizime sodium and injection, which is applied in the field of pharmacy, can solve the problems of increasing the content of impurities, increasing the hidden danger of drug use, and reducing the content of active ingredients, and achieves the effects of simple production process, stable product quality, and easy storage.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
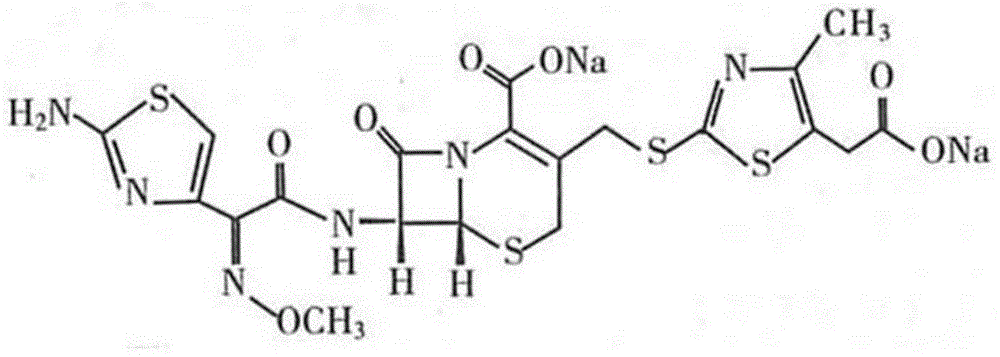
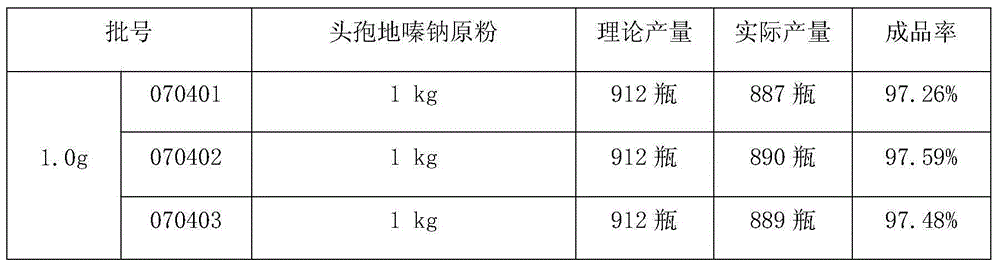
[0021] Example 1 Trial Production of Cefodizime Sodium for Injection
[0022] Prescription: 1.0g / bottle and 2.0g / bottle (in C 20 h 20 N 6 o 7 S 4 count), according to 1000 feeding.
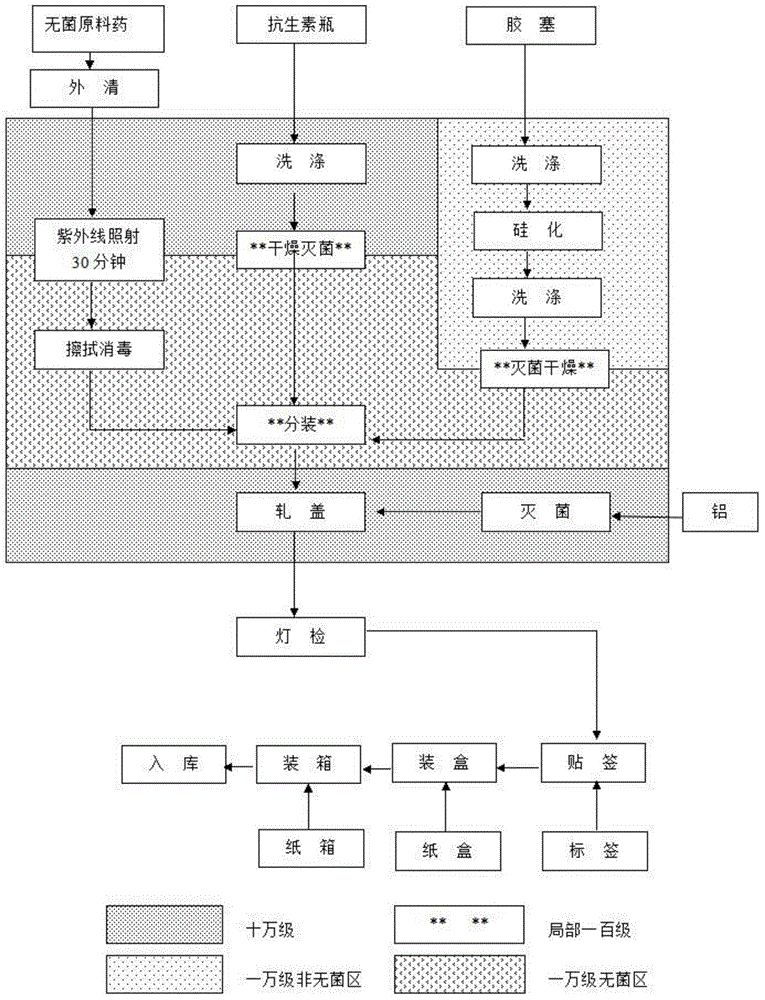
[0023] according to figure 1 The process flow chart shown is for production, and the process is as follows:
[0024] (1) Disassemble the outer packaging of the sterile original powder of cefdizime sodium in the buffer room, first wipe the surface of the original powder bucket (bag) with drinking water, and then wipe and disinfect it with 75% alcohol. Enter the 10,000-level sterile area after being irradiated with ultraviolet light at the transfer window for 30 minutes.
[0025] (2) Soda-lime glass molded injection bottles and halogenated butyl rubber stoppers for sterile powders for injections are washed, dried and sterilized before use.
[0026] (3) Calculate the loading volume according to the converted dry content of the raw material medicine, and adjust the loading volume and machine s...
Embodiment 2
[0034] Example 2 Verification of culture medium simulated packaging process
[0035] Sterile glucose powder was selected as the powder for simulated packaging, and the test medium was trypticase casein soybean broth medium. According to the "Guidelines for the Validation of Drug Production", the contamination rate was 0.10% when the confidence limit was 95% as the qualified standard , select the number of simulated subpackages > 3500 bottles, (that is, the qualified standard is the number of contaminated bottles < 1 bottle).
[0036] 1. Preparation of medium: The medium used for the simulated aliquot test is trypticase casein soybean broth medium (SCMD liquid medium).
[0037] Culture medium sterility test: Divide the sterilized sterile medium for dispensing into 20 sterile test tubes, and culture them at 30-35°C and 20-25°C for 7 days after capping and sealing.
[0038] Test results: After being cultured within 7 days, there was no microbial growth in the culture medium in e...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com