Method for preparing cefodizime sodium
A technology of cefodizime sodium and cefodizime acid, which is applied in the field of drug synthesis, can solve problems such as unfavorable recycling, large environmental pollution, and high price, and achieve the effects of low cost, less environmental pollution, and convenient operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
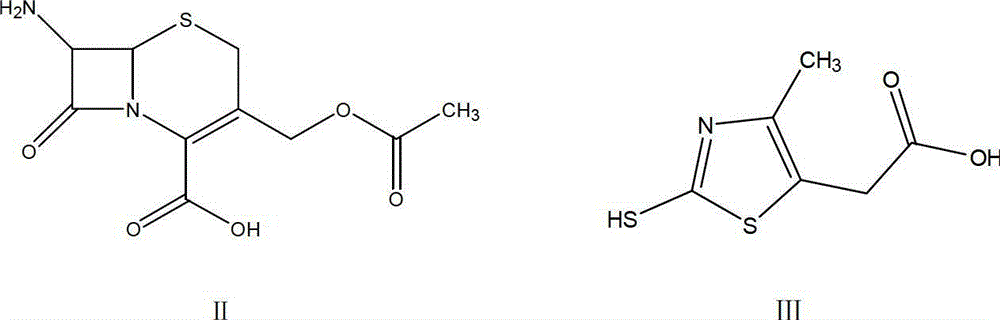
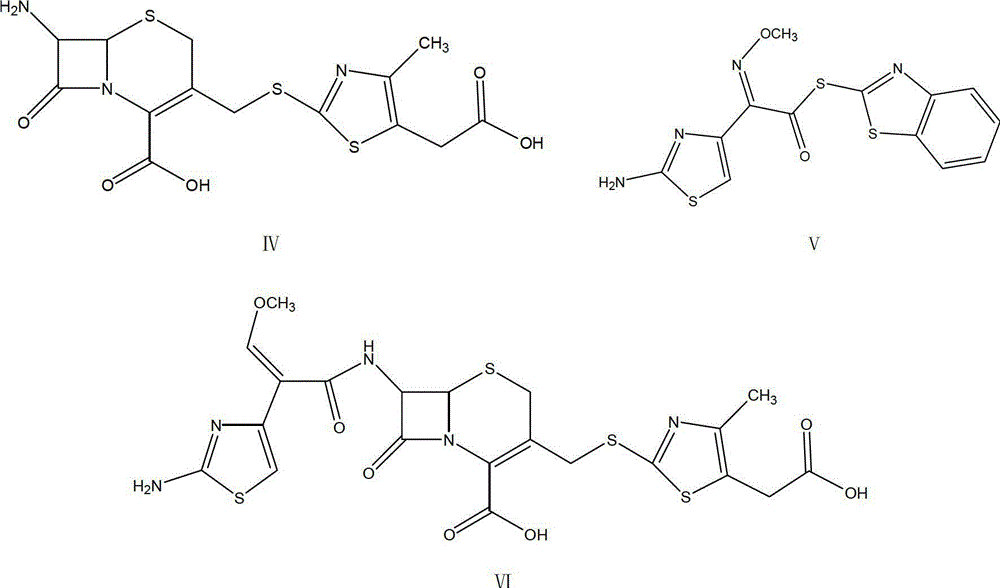
[0044] Preparation of compound of formula IV 7-amino-3-(5-carboxymethyl-4-methyl-1.3-thiazole-2-mercaptomethyl)cephalosporin-2-ene-2-carboxylic acid (TACS)
[0045]
[0046] Add 195ml of acetonitrile solvent to the four-necked flask, then add 22g (0.08mol) of formula II compound 7-ACA and 16g (0.084mol) of formula III compound MMTA, then control the temperature at 28℃~30℃ and add dropwise mass concentration It is 51.8ml of 98% concentrated sulfuric acid. Under the action of concentrated sulfuric acid, 7-ACA and MMTA are reacted. After the dripping is completed, continue to control the temperature and react for 1 hour at 28℃~30℃ to complete the reaction. After the end, HPLC tracking detection is used to determine that the raw material 7-ACA in the reaction solution is less than 1.0%, then the temperature is cooled to 2℃~5℃, and then 100mL ice water and 0.22g sodium hydroxide are added to the reaction solution, and then stirred for 5 minutes. , Control the temperature within 0℃~5℃,...
Embodiment 2
[0048] The compound of formula VI cefodizime acid (6R,7R)-7-[(2-amino-4-thiazolyl)-(methoxyimino)acetamido]-3-[[(5-carboxymethyl-4 -Methyl-2-thiazolyl)thio]methyl]-8-oxo-5-thiabicyclo(4.2.0)oct-2-ene-2carboxylic acid (VI)
[0049]
[0050] Add 26g (0.067mol) of TACS compound of formula IV and 25g (0.07mol) of AE active ester of formula V into another four-necked flask, then add 350ml of dicarboxylic dicarboxylic acid solvent containing 7g of water, and then cool to 0℃~5℃ Then add 18ml of triethylamine, keep the temperature at 0℃~5℃, and react for 4 hours. After the reaction is over, add sulfurous acid to adjust the pH to 5.5, add 180mL of water to extract, extract three times, and combine the aqueous phase of the three extractions. Then add 2.6g of egret activated carbon to the water phase for 30 minutes, filter, wash the filter cake with 20mL of water, collect the filtrate, then adjust the pH value to 2.8 with 3mol / L sulfuric acid aqueous solution, after the adjustment, stir to ...
Embodiment 3
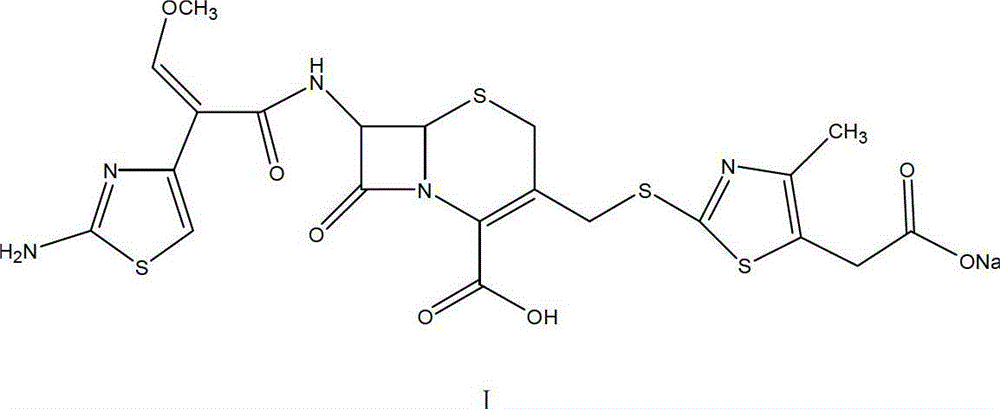
[0052] The preparation of formula I compound cefodizime sodium
[0053] Add 40mL ethanol solvent into the four-neck flask, add a total of 24g cefodizime acid in three batches, then cool down, control the temperature at 0℃~3℃, dropwise add salt-forming agent sodium bicarbonate and sodium carbonate mixed aqueous solution (pH The value is 8.9~9.1), the amount of the mixed solution added is to dissolve all the cefodizime acid, and control the pH value of the system reaction solution to be less than 8.0, then add 2.4g egret activated carbon, decolorize for 30 minutes, filter, Collect the filtrate, wash the filter cake with 10 mL of 67% ethanol aqueous solution, combine the filtrate, control the temperature at 15 ℃ to 20 ℃, add 350 mL of ethanol dropwise, after the addition is complete, stir for 30 minutes and filter to obtain a solid wet product. It was dried under vacuum to obtain 22.9 g of cefodizime sodium as the final product, with a yield of 88%, HPLC purity: 99.7%, and moisture ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com