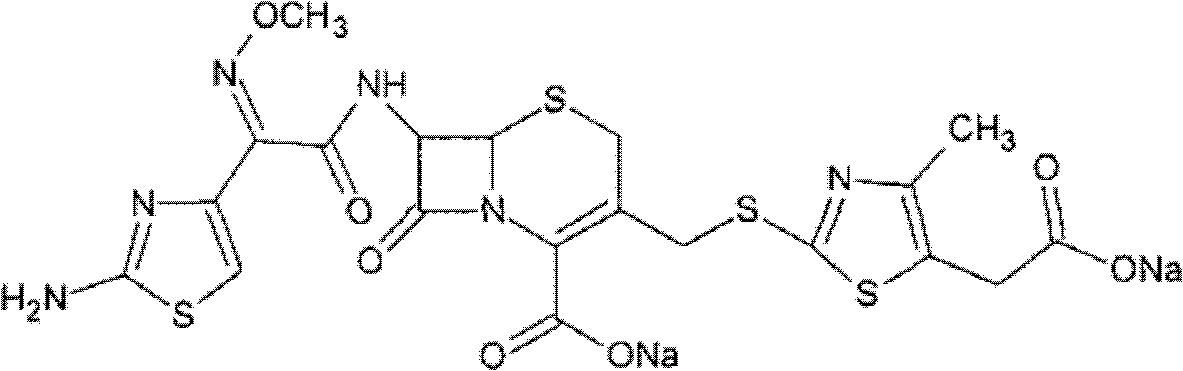
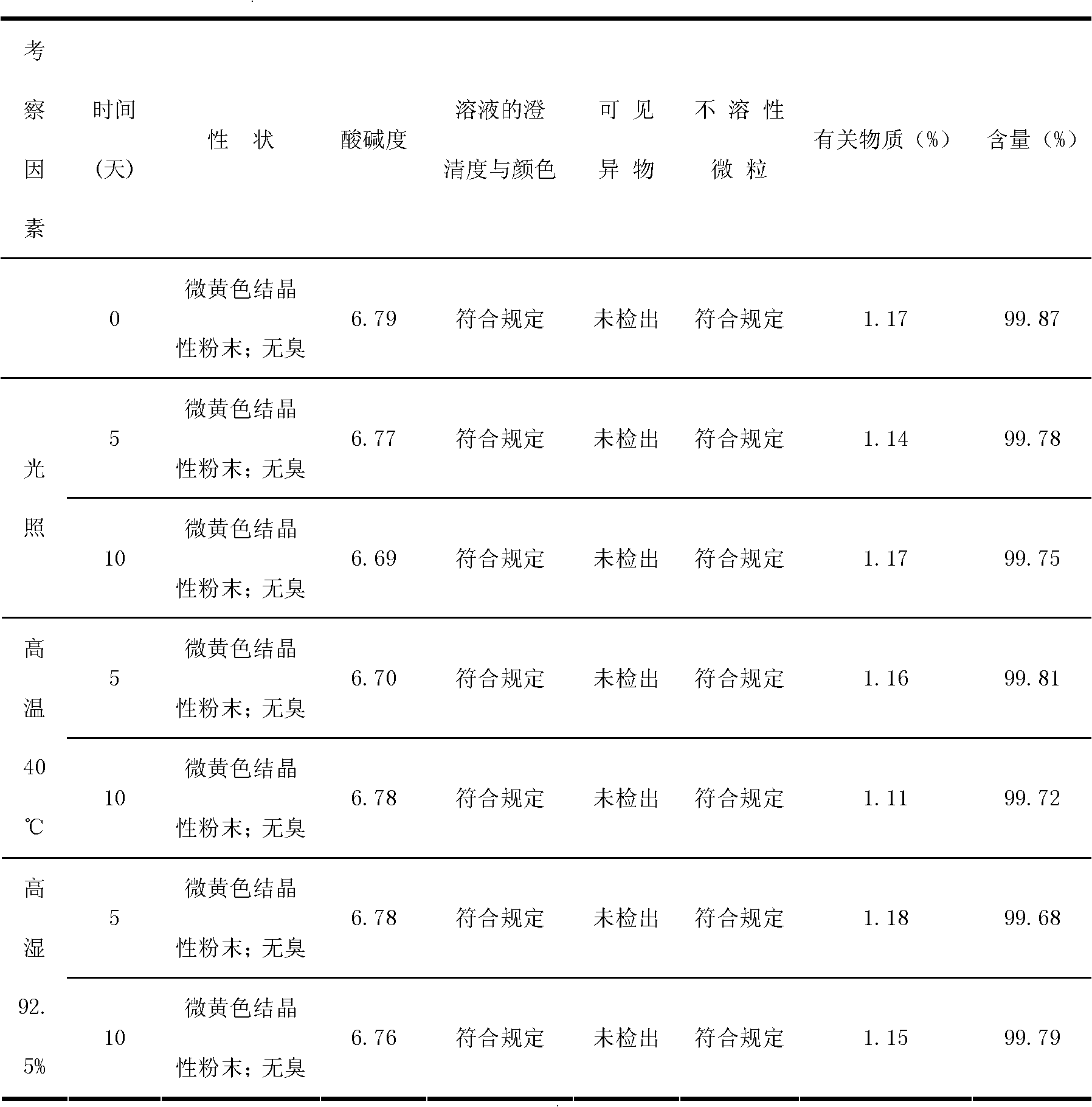
Cefodizime sodium composition and powder injection
A technology of cefidiazine and composition, which is applied in the field of medicine and can solve problems such as the difference in dissolution rate and bioavailability of preparations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Embodiment 1: the preparation of cefodizime sodium crystal
[0029] Dissolve the solid cefodizime sodium in distilled water, control the pH value of the solution to 6.6-7.2, and add a mixed solvent of anhydrous isopropanol, anhydrous ethanol and anhydrous diethyl ether at 15°C at a rate of 6ml / minute; the volume ratio of anhydrous isopropanol, absolute ethanol and anhydrous ether is 1:1.25:0.5, and the stirring speed is 100 rpm; after the mixed solvent is added, the temperature is lowered to 4°C; after crystals are obtained, continue to stir for 2 hours , stirring at a speed of 65 rpm; filtering, washing the filter cake with ethanol for 2 to 3 times, and vacuum drying for 2 hours to obtain cefodizime sodium crystals.
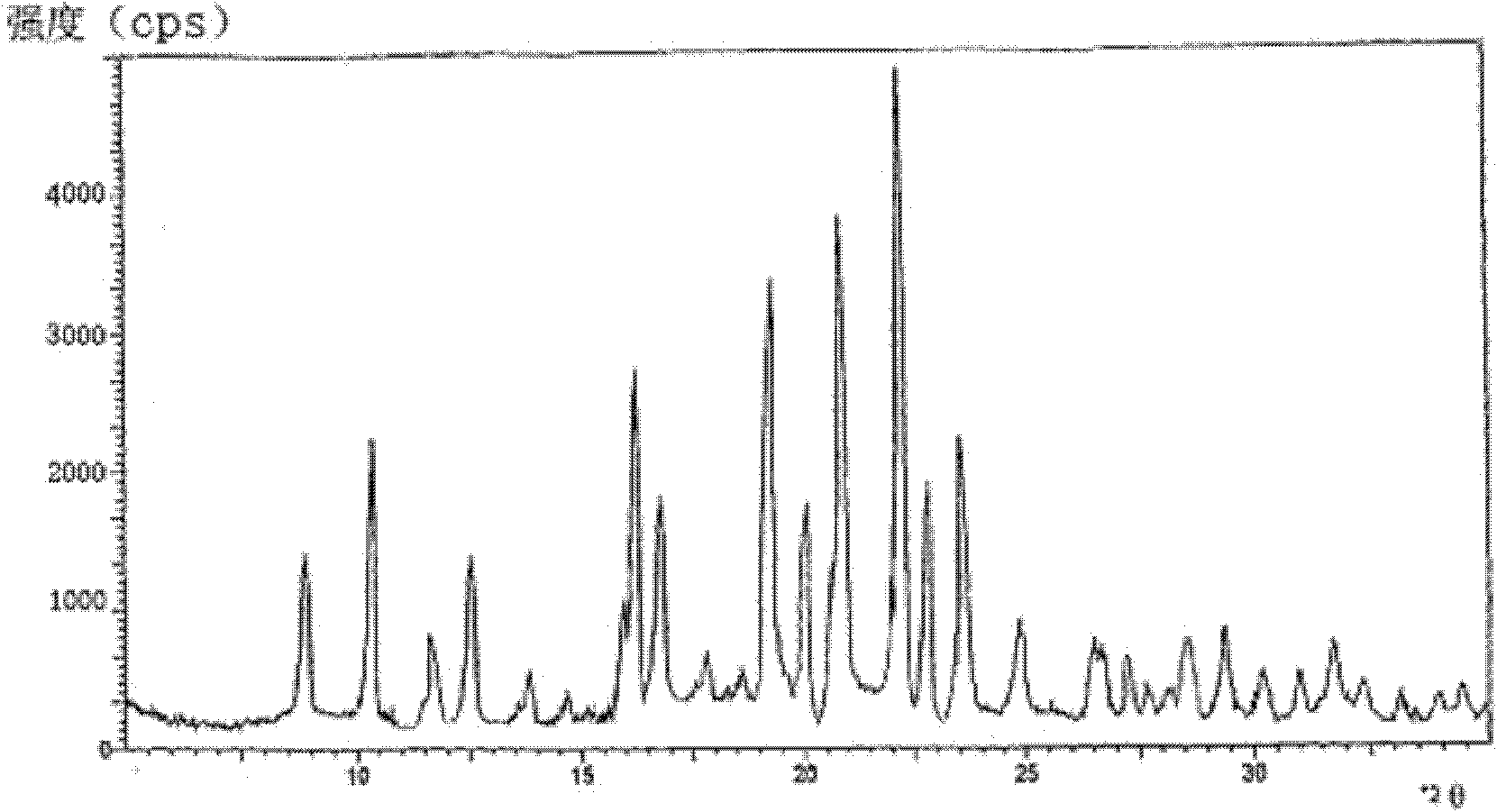
[0030] The X-ray powder diffraction pattern obtained by measuring Cu-Kα rays of the crystal is as follows: figure 1 shown.
Embodiment 2
[0031] Embodiment 2: the preparation of cefodizime sodium crystal
[0032] Dissolve the solid cefodizime sodium in distilled water, control the pH value of the solution to 6.6-7.2, and add a mixed solvent of water isopropanol, absolute ethanol and anhydrous ether under the condition of 20°C, and the dropping rate is 8ml / min; anhydrous The volume ratio of isopropanol, absolute ethanol and anhydrous ether is 1: 1.75: 0.75, and the stirring speed is 120 rpm; after the mixed solvent is added, the temperature is lowered to 10°C, and stirring is continued for 4 hours after the crystal is obtained, and the stirring speed is 75 RPM; filter, wash the filter cake with ethanol, and vacuum-dry for 4 hours to obtain cefodizime sodium crystals. Show through powder XRD detector analysis, with attached figure 1 The results shown match.
Embodiment 3
[0033] Embodiment 3: the preparation of cefodizime sodium crystal
[0034] Dissolve the solid cefodizime sodium in distilled water, control the pH value of the solution to 6.6-7.2, add dropwise a mixed solvent of water isopropanol, absolute ethanol and anhydrous ether at 15°C, and the dropping speed is 7ml / min The volume ratio of anhydrous isopropanol, absolute ethanol and anhydrous ether is 1: 1.5: 0.7, and the stirring speed is 110 rpm; after the mixed solvent is added, the temperature is lowered to 10 ° C, and the crystal is obtained and then stirred for 3 hours. The stirring speed was 70 rpm; filtered, the filter cake was washed with ethanol, and vacuum-dried for 3 hours to obtain cefodizime sodium crystals. Show through powder XRD detector analysis, with attached figure 1 The results shown match.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com