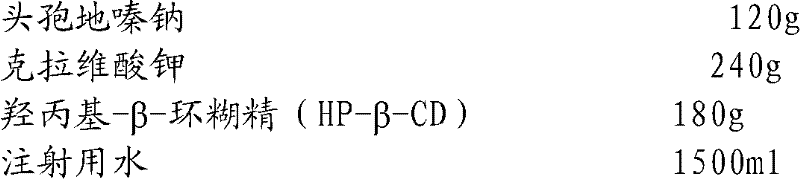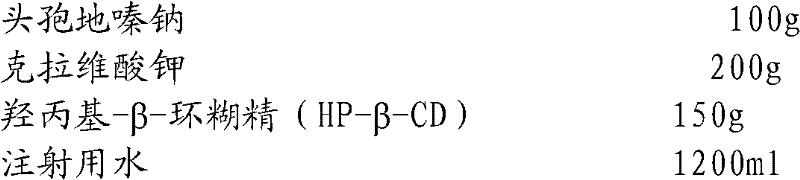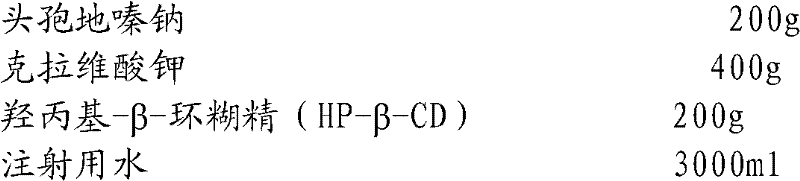Cefodizime sodium composition and preparation method thereof
A technology of cefodizime sodium and its composition, which is applied in the field of stable cefodizime sodium composition and its preparation, can solve the problems of insufficient antibacterial spectrum of cefodizime sodium, failure to meet quality requirements, increase of related substances, etc., and achieve prescription The process is simplified, the efficacy of the drug is not changed, and the safe dose is large.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] The proportioning of composition is:
[0040]
[0041] Preparation Process:
[0042]Add sterile cefodizime sodium to the reaction kettle at room temperature and dissolve it in water for injection; add sterile potassium clavulanate, stir to make it completely dissolve, and obtain the aqueous solution of the mixture of cefodizime sodium and clavulanate potassium; Add hydroxypropyl-β-cyclodextrin (HP-β-CD) to the mixture, stir and clathrate for 1.0 hour; add water for injection to dilute to the required content of cefodizime sodium preparation for injection, and then use a 0.45 μm microporous filter Membrane filtration, and finally filtration through a 0.22 μm microporous membrane, subpackaging, and freeze-drying to obtain cefodizime sodium / clavulanate potassium freeze-dried powder for injection.
Embodiment 2
[0044]
[0045] Preparation Process:
[0046] Add sterile cefodizime sodium to the reaction kettle at room temperature and dissolve in water for injection; add sterile L-clavulanate potassium, stir to make it all dissolve, and obtain the aqueous solution of cefodizime sodium and clavulanate potassium mixture; Add hydroxypropyl-β-cyclodextrin (HP-β-CD) into the reaction kettle, stir and clathrate for 1.5 hours; then filter through a 0.45 μm microporous membrane, and finally filter through a 0.22 μm microporous membrane, and separate Pack, obtain cefodizime sodium injection.
Embodiment 3
[0048]
[0049] Preparation Process:
[0050] Add sterile cefodizime sodium to the reaction kettle at room temperature and dissolve it in water for injection; add sterile potassium clavulanate, stir to make it completely dissolve, and obtain the aqueous solution of the mixture of cefodizime sodium and clavulanate potassium; Add hydroxypropyl-β-cyclodextrin (HP-β-CD) to the mixture, stir and clathrate for 0.5 hour; add water for injection to dilute to the required content of cefodizime sodium preparation for injection, and then use a 0.45 μm microporous filter Membrane filtration, and finally filtration through a 0.22 μm microporous membrane, subpackaging, and freeze-drying to obtain cefodizime sodium / clavulanate potassium freeze-dried powder for injection.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com