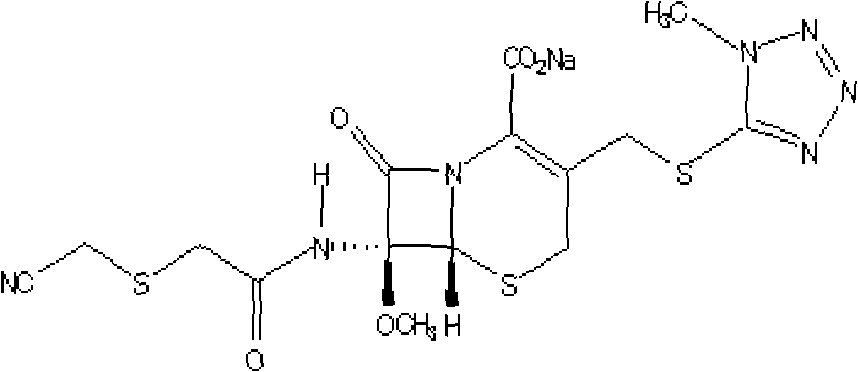
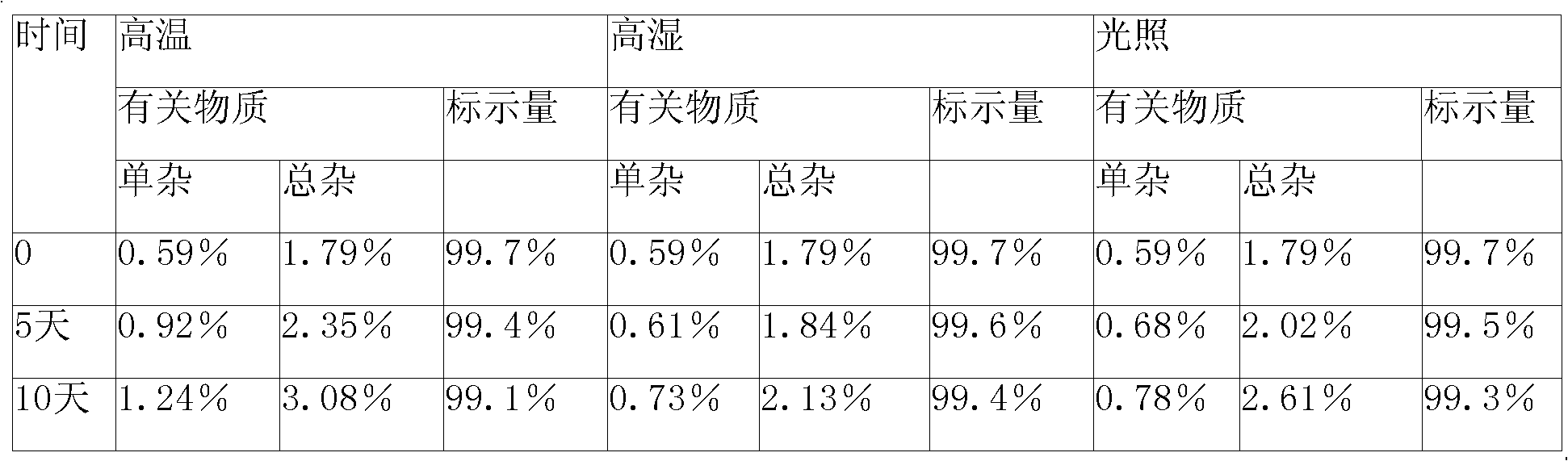
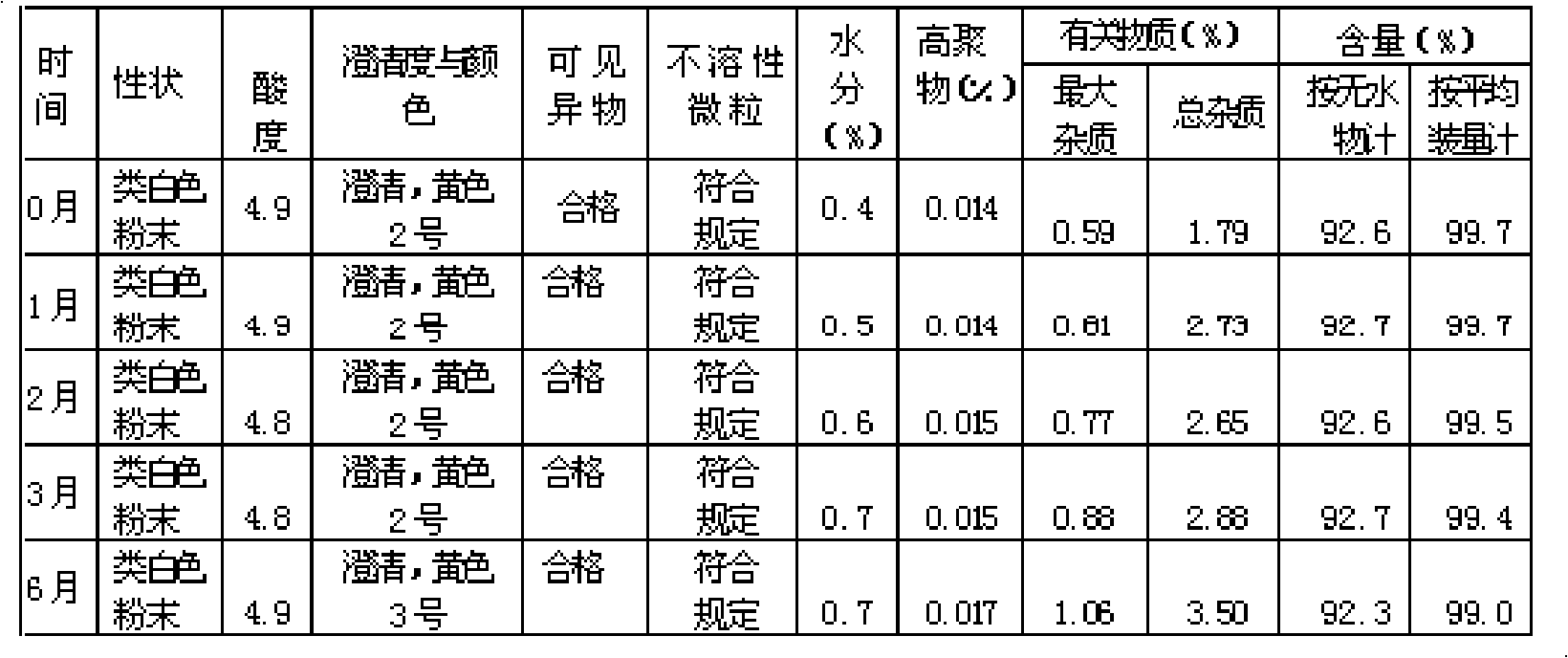
Cefmetazole sodium medicament and preparation method thereof
A technology for cefmetazole sodium and medicine, which is applied to the field of cefmetazole sodium medicine and its preparation, can solve the problems of poor clarity, slow reconstitution, poor uniformity of mixed powder, etc., and achieves reduction of difference in loading and irritation. , the effect of improving liquidity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0068] Specification: 0.25g (in C 15 h 17 N 7 o 5 S 3 count)
[0069] prescription:
[0070] Cefmetazole sodium 250g (in C 15 h 17 N 7 o 5 S 3 count)
[0071] A total of 1000 bottles were made
[0072] Preparation Process:
[0073] (1) Pretreatment
[0074] a) In a class 10,000 clean area, add cefmetazole sodium into the water for injection, slowly raise the temperature to 40°C, stir to dissolve, add medicinal charcoal, stir, filter while hot to remove charcoal, then filter through a filter membrane, and cool to room temperature, add ethanol and mix evenly, stir and crystallize for 12 hours to obtain a crystallization solution;
[0075] b) transfer the crystallization solution to a sterile room, filter, wash with ethanol, filter, and dry to obtain a sterile powder of cefmetazole sodium;
[0076] (2) Aseptic packaging
[0077] The cefmetazole sodium aseptic powder obtained in the last step is subpackaged to antibiotic glass bottles under aseptic conditions, and ...
Embodiment 2
[0079] Specification: 0.5g (in C 15 h 17 N 7 o 5 S 3 count)
[0080] prescription:
[0081] Cefmetazole sodium 500g (in C 15 h 17 N 7 o 5 S 3 count)
[0082] A total of 1000 bottles were made
[0083] Preparation Process:
[0084] (1) Pretreatment
[0085] a) In a class 10,000 clean area, add cefmetazole sodium into the water for injection, slowly raise the temperature to 40°C, stir to dissolve, add medicinal charcoal, stir, filter while hot to remove charcoal, then filter through a filter membrane, and cool to room temperature, add ethanol and mix evenly, stir and crystallize for 35 hours to obtain a crystallization solution;
[0086] b) transfer the crystallization solution to a sterile room, filter, wash with ethanol, filter, and dry to obtain a sterile powder of cefmetazole sodium;
[0087] c) extruding and pre-crushing the sterile cefmetazole sodium powder obtained in the previous step with a ball mill, and pulverizing it into a fine powder that all passes ...
Embodiment 3
[0091] Specification: 1.0g (in C 15 h 17 N 7 o 5 S 3 count)
[0092] prescription:
[0093] Cefmetazole sodium 1000g (in C 15 h 17 N 7 o 5 S 3 count)
[0094] A total of 1000 bottles were made
[0095] Preparation Process:
[0096] (1) Pretreatment
[0097] a) In a class 10,000 clean area, add cefmetazole sodium into the water for injection, slowly raise the temperature to 40°C, stir to dissolve, add medicinal charcoal, stir, filter while hot to remove charcoal, then filter through a filter membrane, and cool to room temperature, add ethanol and mix evenly, stir and crystallize for 20 hours to obtain a crystallization solution;
[0098] b) transfer the crystallization solution to a sterile room, filter, wash with ethanol, filter, and dry to obtain a sterile powder of cefmetazole sodium;
[0099] c) extruding and pre-crushing the sterile cefmetazole sodium powder obtained in the previous step with a ball mill, and pulverizing it into a fine powder that all passes...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com