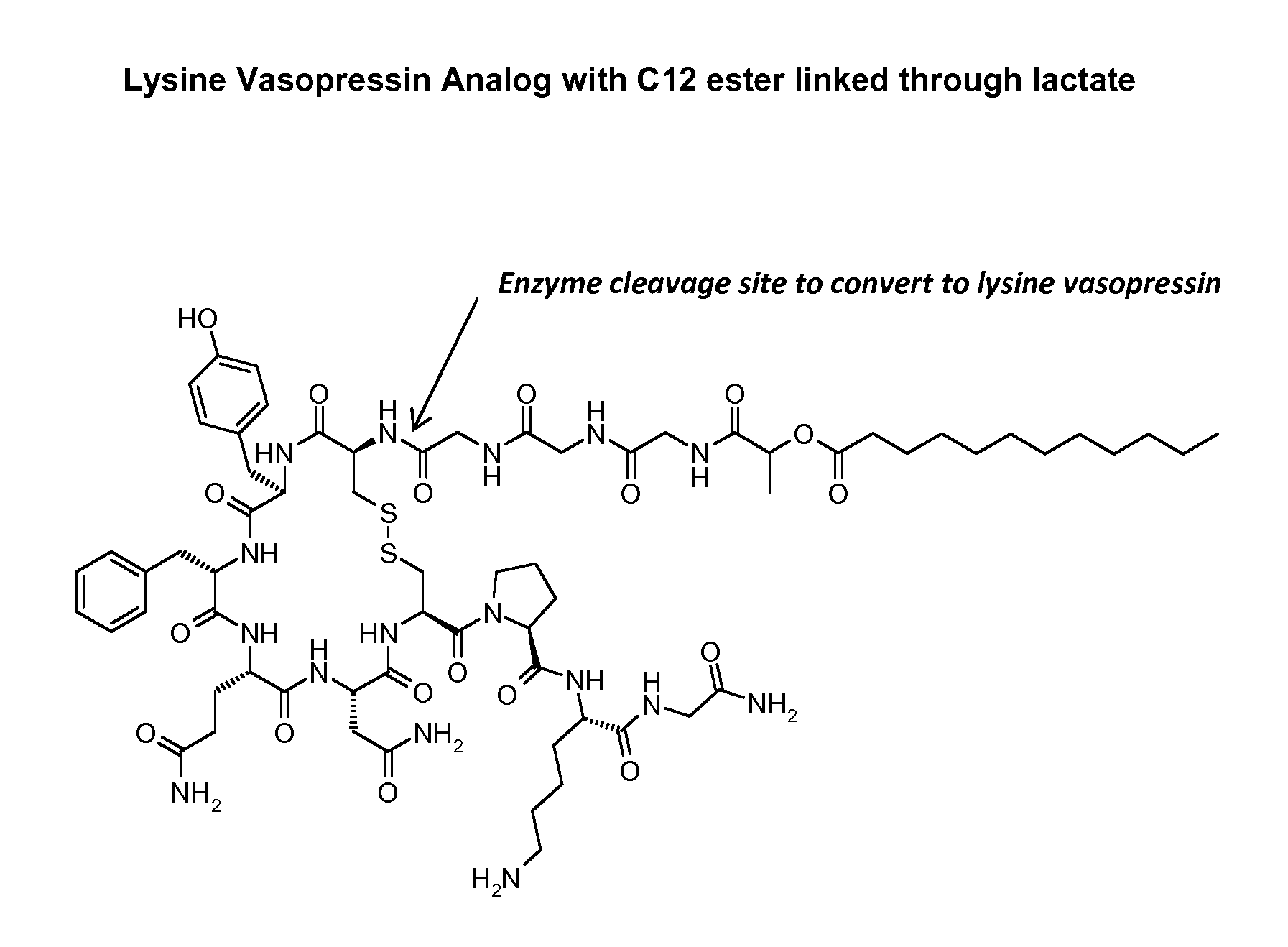
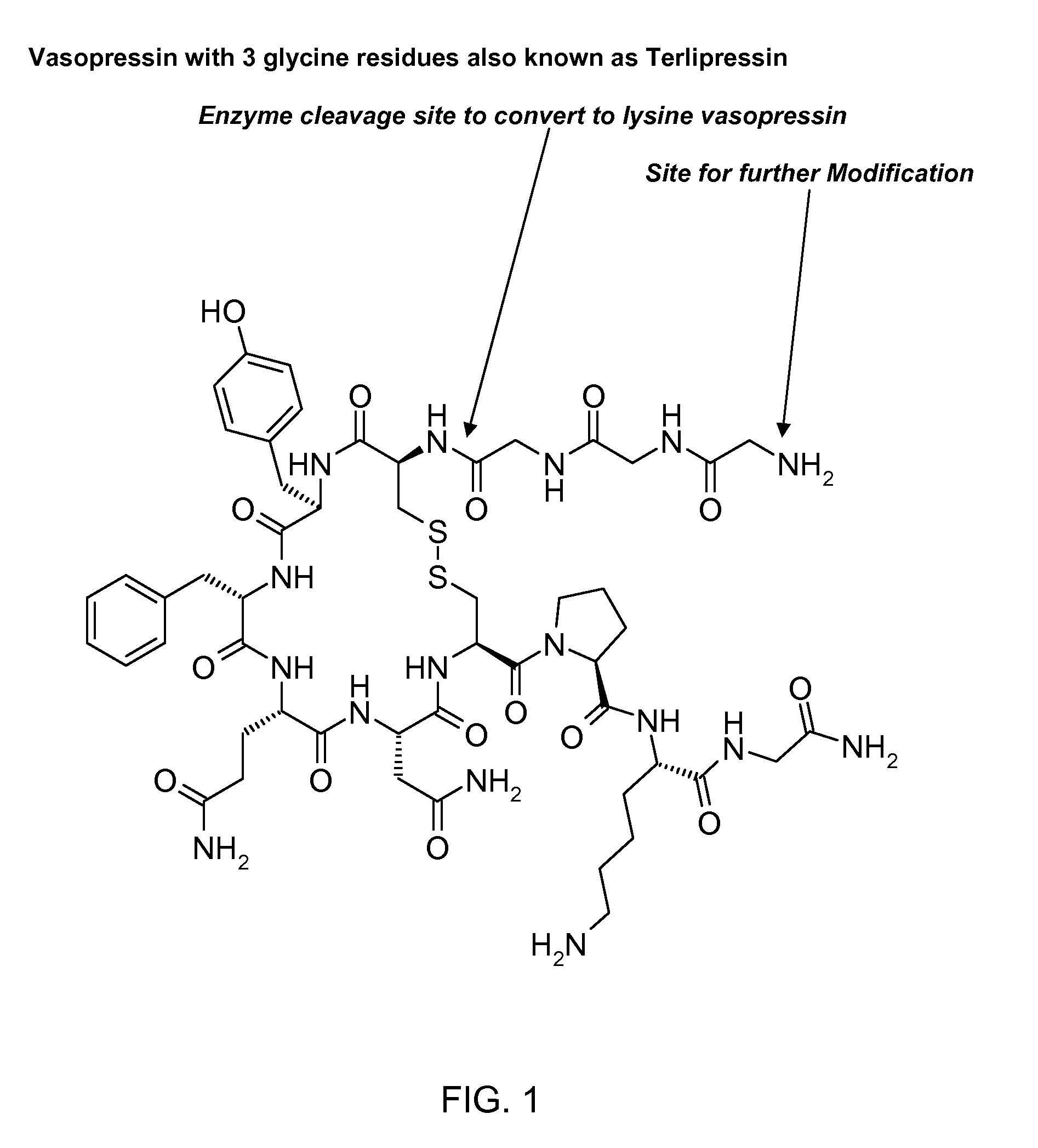
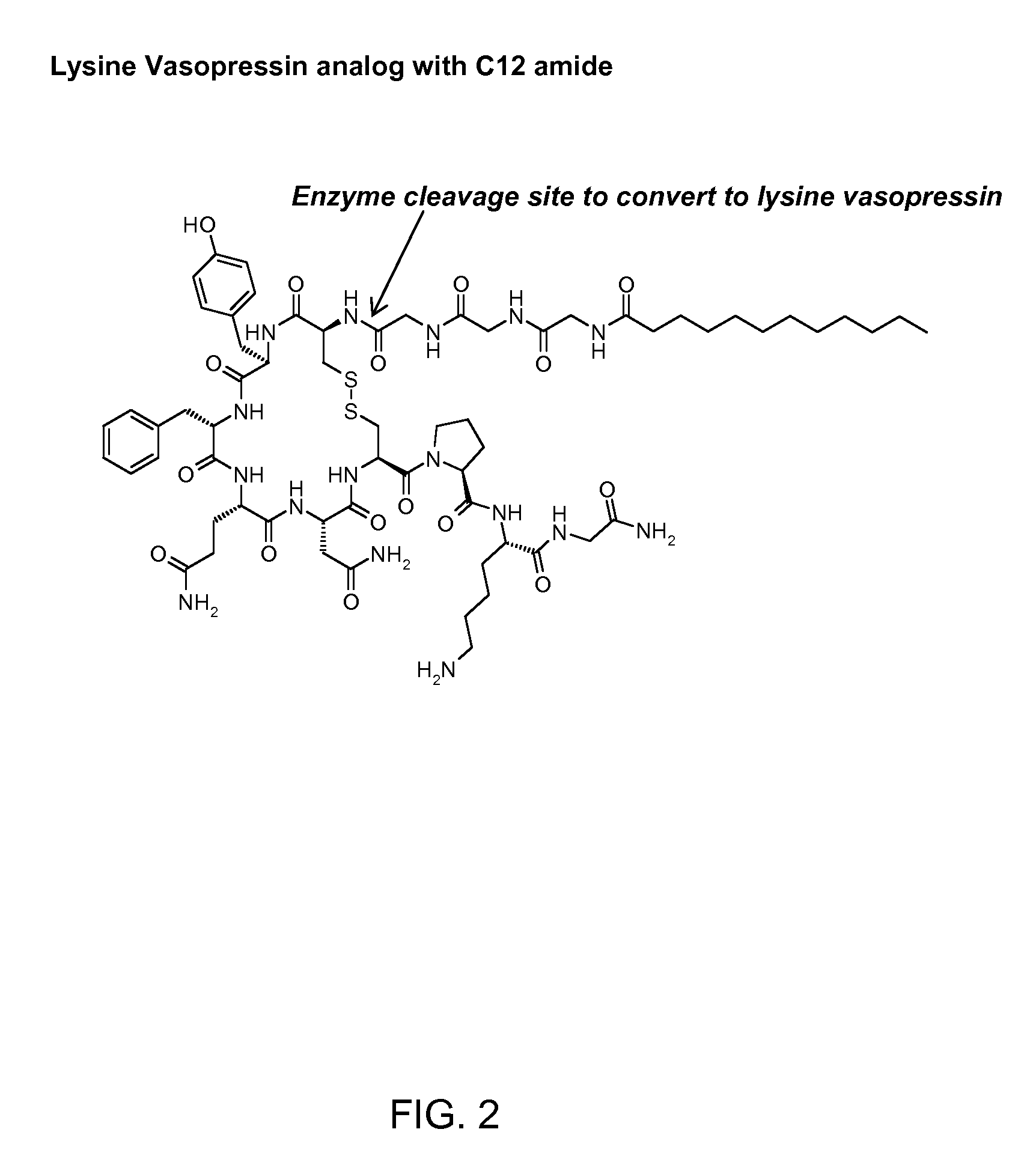
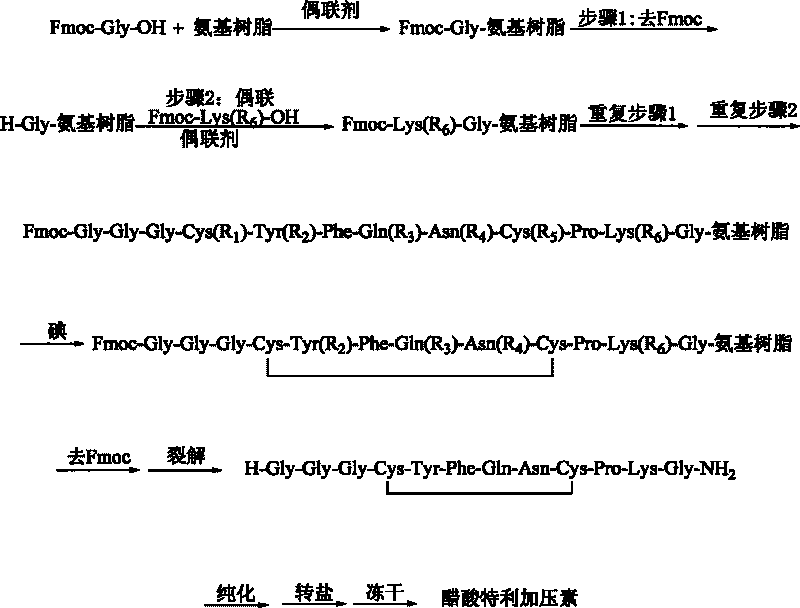
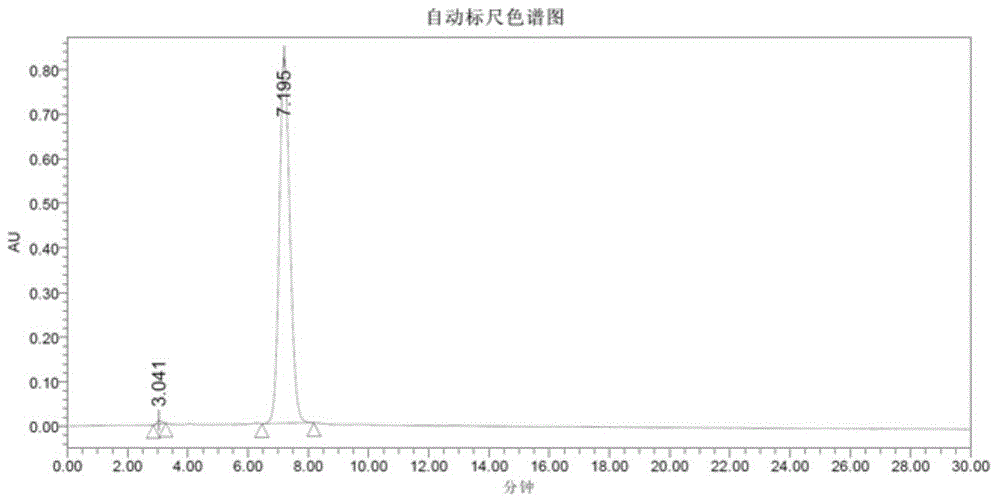
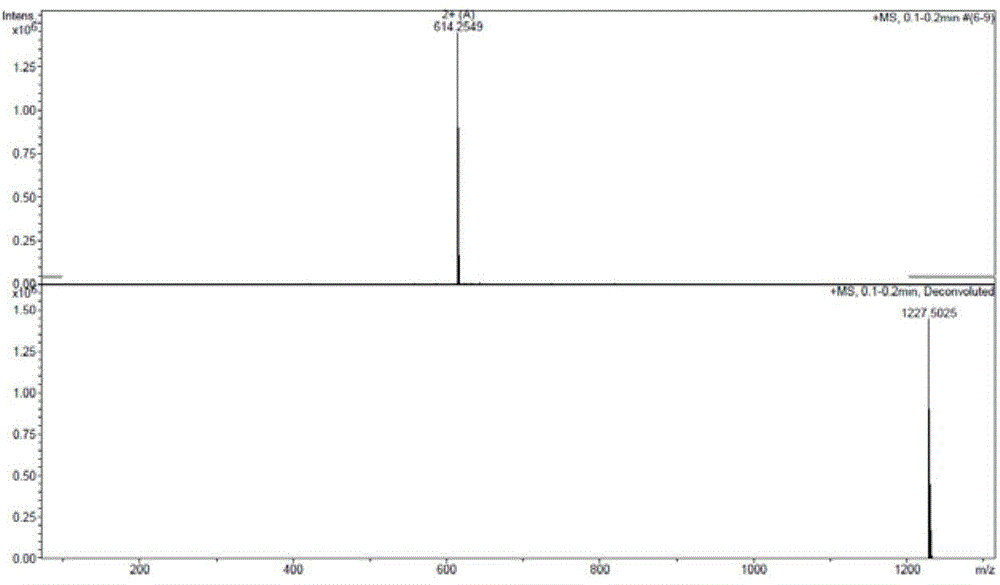
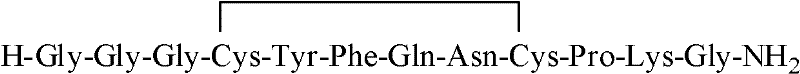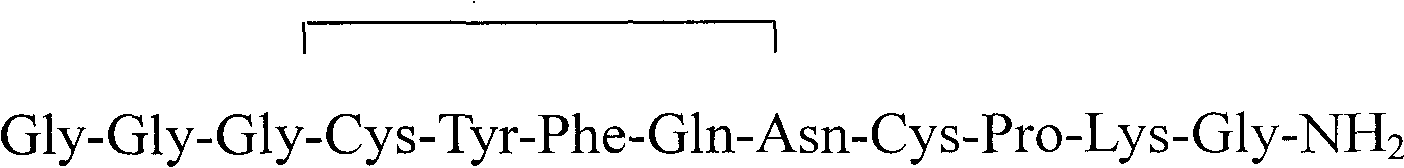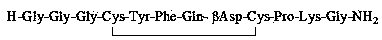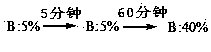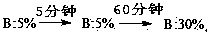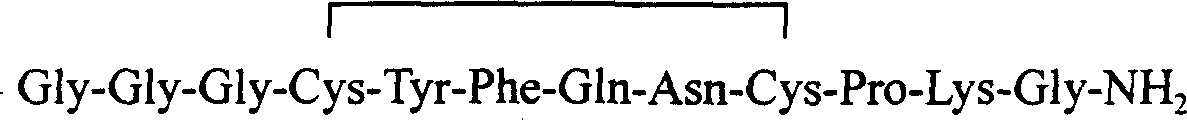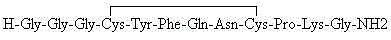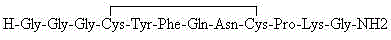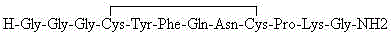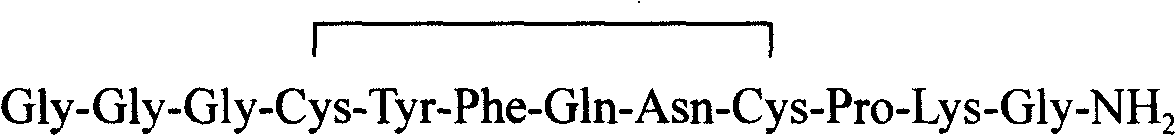Patents
Literature
38 results about "Terlipressin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
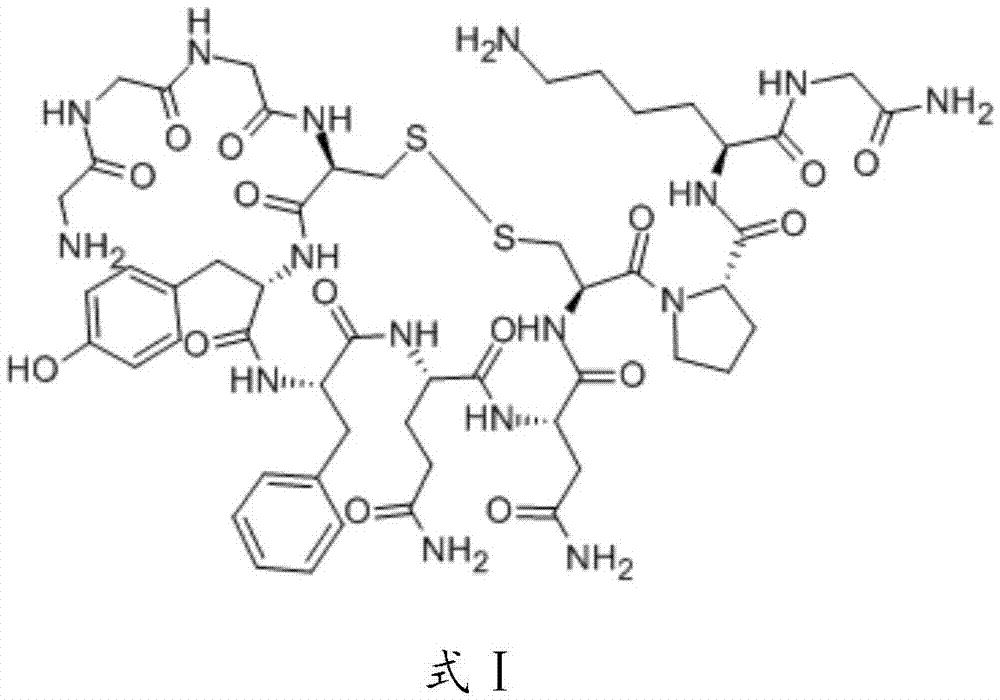
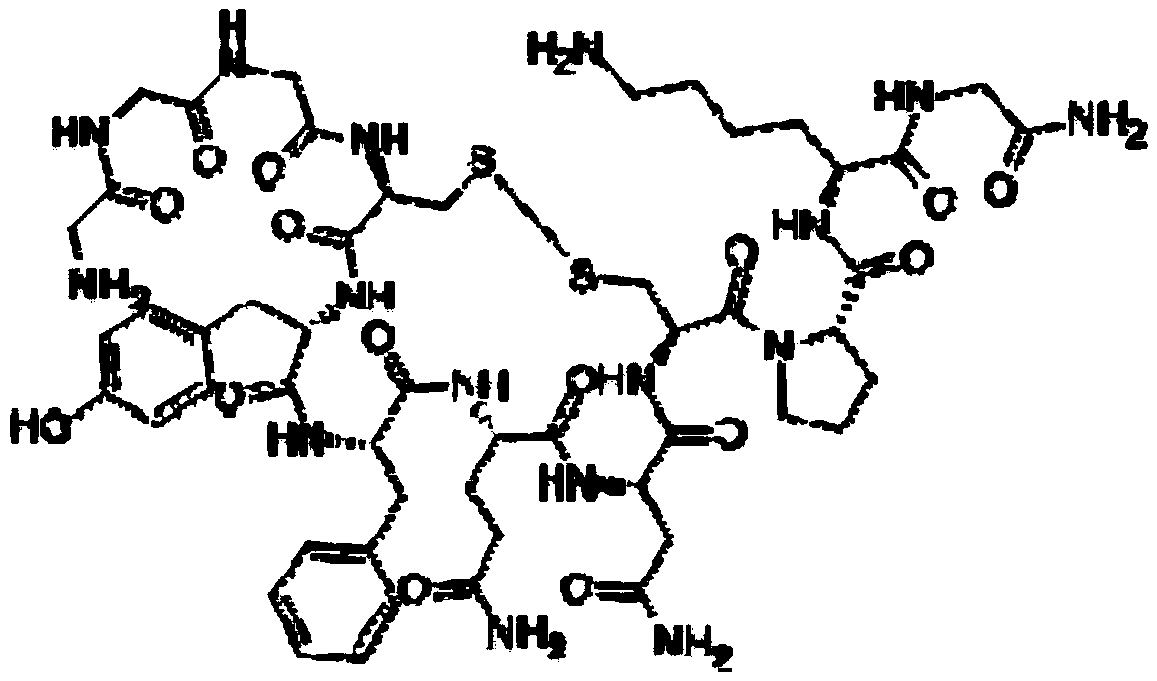
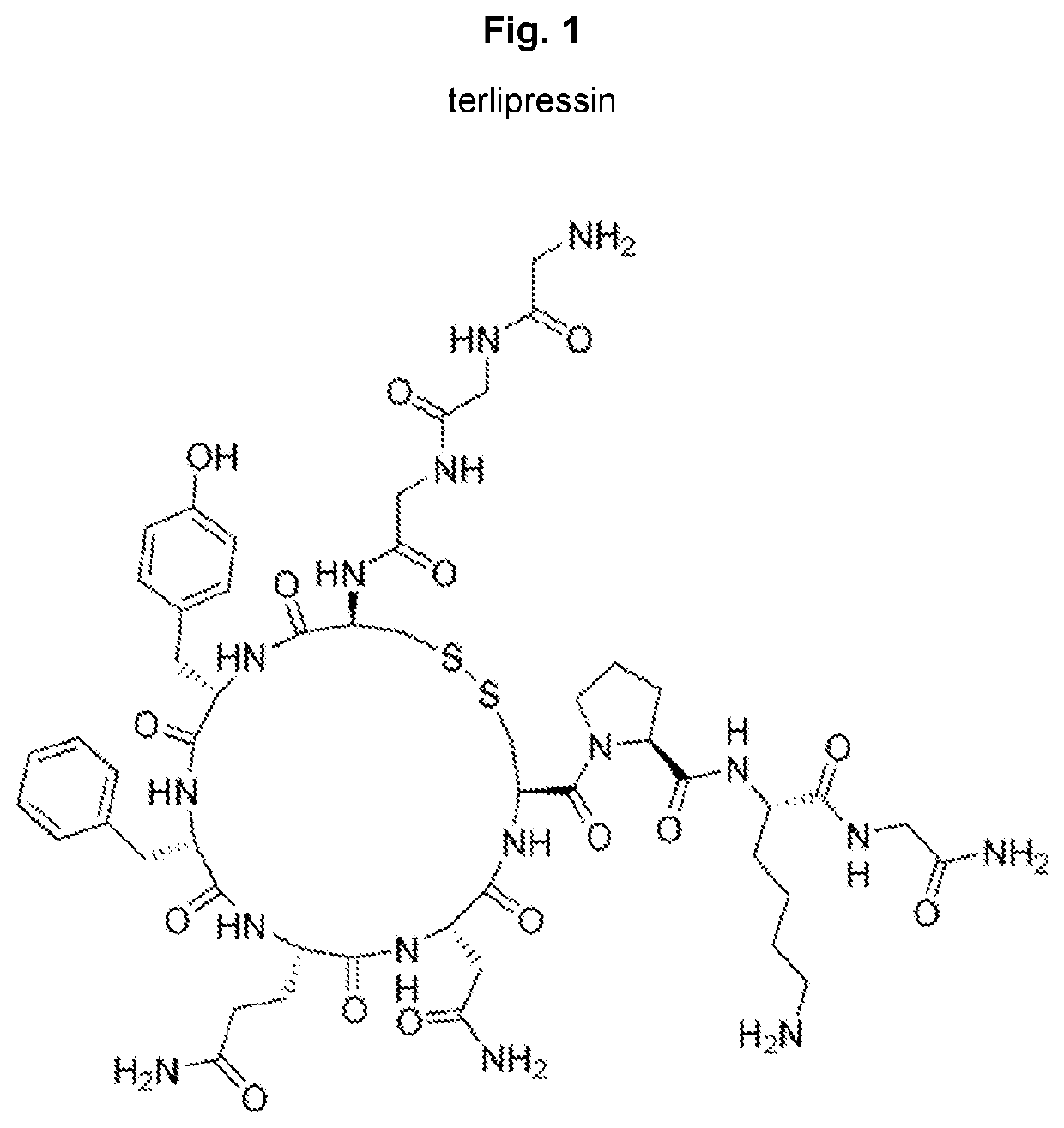
Terlipressin (trade names Teripress by New Medicon Pharma and Glypressin by Ferring Pharmaceuticals) is an analogue of vasopressin used as a vasoactive drug in the management of low blood pressure. It has been found to be effective when norepinephrine does not help.
Composition for long-acting peptide analogs
ActiveUS7960336B2Increase perfusionImprove the level ofAntibacterial agentsPeptide/protein ingredientsHalf-lifeArginine
Owner:PHARMAIN CORP
Method for improving stability of polypeptide active pharmaceutical ingredients
ActiveCN106188218AChange particle shapeChange moistureOxytocins/vasopressinsThymosin peptidesOxytocinBivalirudin
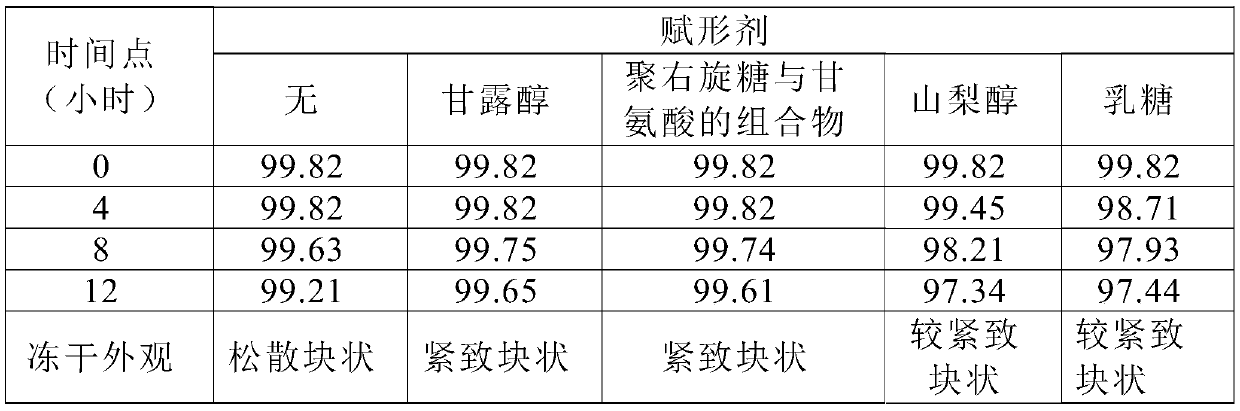
The invention discloses a method for improving the stability of polypeptide active pharmaceutical ingredients. The polypeptide active pharmaceutical ingredients comprise but are not limited to bivalirudin, octreotide acetate, lanreotide acetate, eptifibatide or cetrorelix acetate, ganirelix acetate, degarelix, liraglutide, oxytocin, thymosin alpha1, leuprolide acetate, goserelin acetate, terlipressin or linaclotide. The method comprises the steps that after polypeptide drugs are salified, a polypeptide solution containing compensation ions is obtained, and a target polypeptide product is prepared through an ultralow temperature vacuum freeze-drying method. According to the method for improving the stability of the polypeptide active pharmaceutical ingredients, the problem that the polypeptide active pharmaceutical ingredients are prone to degradation after being placed for a long time is solved, the uniformity of the product is improved, and the drug risk is reduced.
Owner:SINOPEP ALLSINO BIOPHARMACEUTICAL CO LTD
Method for synthesizing terlipressin by solid-phase oxidization and cyclization
ActiveCN101693738AReduce dosageHigh purityOxytocins/vasopressinsPeptide preparation methodsFreeze-dryingTerlipressin
The invention discloses a method for synthesizing terlipressin by solid-phase oxidization and cyclization. The method comprises the following steps: (1) obtaining Fmoc-Gly-amino resins by using Fmoc-Gly-OH and amino resins; (2) coupling the Fmoc-Gly-amino resins one by one to obtain linear terlipressin-amino resins; (3) synthesizing the linear terlipressin-amino resins into terlipressin-amino resins by adopting iodine solid-phase oxidized cyclization; (4) cracking and cutting the terlipressin-amino resins to obtain raw peptides of the terlipressin; and (5) carrying out purification, salt conversion and freeze-drying on the raw peptides to obtain terlipressin acetate. The invention has the key innovations that solid-phase oxidization reduces a large amount of waste liquid brought by liquid-phase oxidization, accelerates the reaction time, belongs to process greening reformation and greatly improves the total yield.
Owner:HYBIO PHARMA
Preparation method of Terlipressin
InactiveCN102408471AHigh yieldShorten the production cycleOxytocins/vasopressinsPeptide preparation methodsSynthesis methodsGly-Gly-Gly
The invention belongs to the technical field of preparation methods of polypeptide drugs and particularly relates to a preparation method of Terlipressin. The method uses protected amino acid fragment Gly-Gly-Gly as a raw material to avoid the generation of impurities. The method comprises the following steps: preparing Terlipressin linear peptide resin by adopting a solid phase polypeptide method, performing acidolysis to obtain crude Terlipressin linear peptide, oxidizing to obtain crude Terlipressin and purifying to obtain pure Terlipressin, wherein the solid phase polypeptide method is as follows: amino resin is connected in the Fmoc-protected amino acid of the corresponding fragments in the following sequence in turn through a solid phase coupling synthesis method to prepare the Terlipressin linear peptide resin, the sequence is R1-X-Cys(R2)-Tyr(tBu)-Phe-Gln(R3)-Asn(R3)-Cys(R2)-Pro-Lys(Boc)-Gly-resin, the fragment X is Gly-Gly-Gly, R1 is R4 or H, R2 is Trt or Acm, R3 is Trt or H, R4 is Fmoc or Boc, and the fragment X is grafted only by performing the solid phase coupling synthesis reaction once. The method uses the new raw material, thus the product yield can be increased and the production cycle is greatly shortened.
Owner:CHENGDU SHENGNUO BIOTEC CO LTD
Terlipressin preparation and preparations method thereof
InactiveCN102068685AImprove stabilityLong durationPowder deliveryPeptide/protein ingredientsMedicineActive component
The invention discloses a terlipressin preparation and a preparations method thereof, belonging to the field of medical preparations. The terlipressin preparation comprises active components and auxiliary materials; the active components include terlipressin and pharmaceutically acceptable salts; the auxiliary materials include excipient and a pH regulator; and the weight part ratio of the terlipressin to the excipient to the pH regulator is (1-50):(1-1000):(1-200). Because the terlipressin is easy to degrade under the condition of acid or base, found by research of the invention, the stability of the active components in the terlipressin preparation can be greatly enhanced by selecting a proper pH range. With the pH range determined in the proportion range of the active components to the excipient to the pH regulator determined by the invention, researched by stability test, the stability of the active components is greatly enhanced, and the drug effect period of the active components is greatly prolonged under the same storage condition.
Owner:HYBIO PHARMA
Purifying method for terlipressin
ActiveCN103992391AHigh purityHigh yieldOxytocins/vasopressinsPeptide preparation methodsDesalinationFreeze-drying
The invention relates to a purifying method for terlipressin. The method is characterized by comprising the following steps: step 1, dissolving a crude terlipressin product with water and adjusting a pH value to 3 to 4; step 2, flushing a C18 reversed-phase filled column by using an ion pair mobile phase A containing surfactant; step 3, loading a solution obtained in the step 1 into a C18 reversed-phase filling material; step 4, carrying out gradient procedures, wherein the initial-state mobile phase B of an elution gradient is 5% which is maintained for 5 min, then the proportion of the mobile phase B is increased to 30% within 60 min, and a part of target peak elution fraction is collected; and step 5, carrying out nanofiltration membrane desalination and then freeze drying so as to obtain pure terlipressin.
Owner:ADLAI NORTYE BIOPHARMA CO LTD
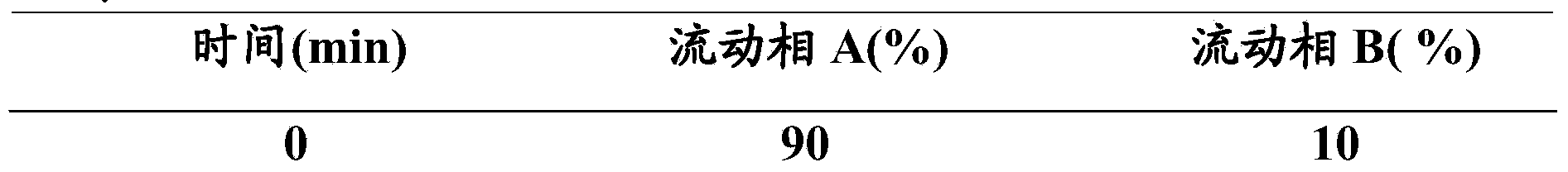
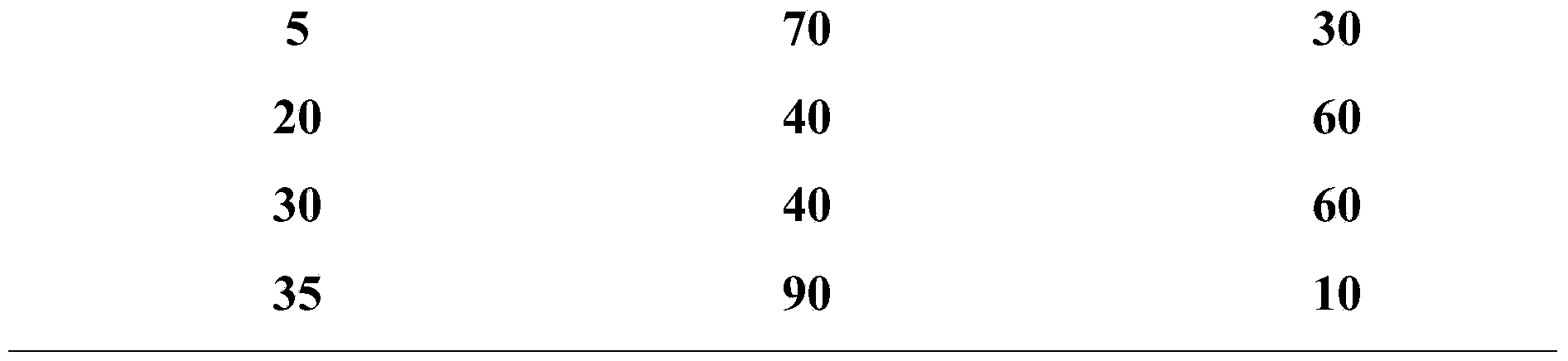
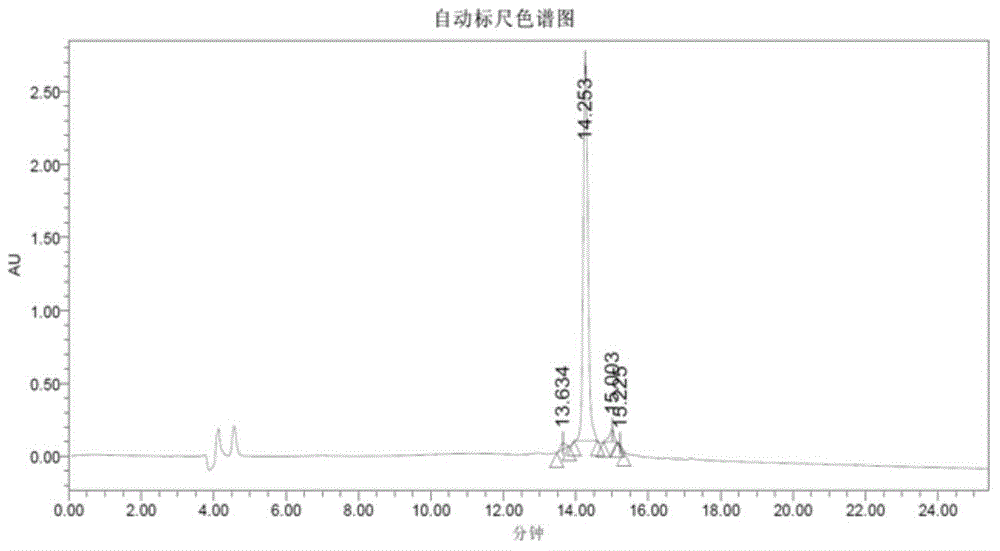
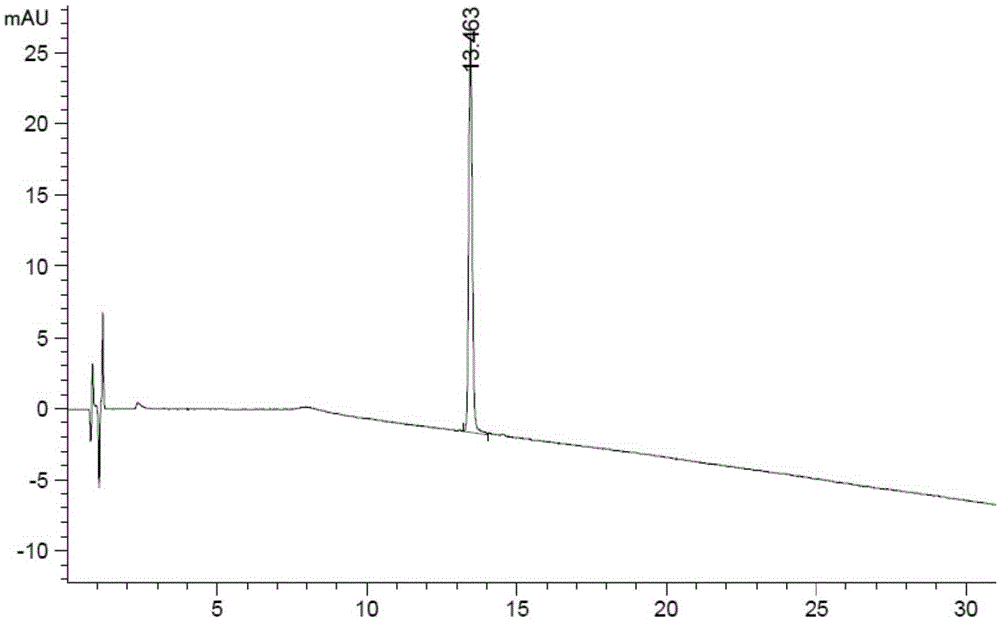
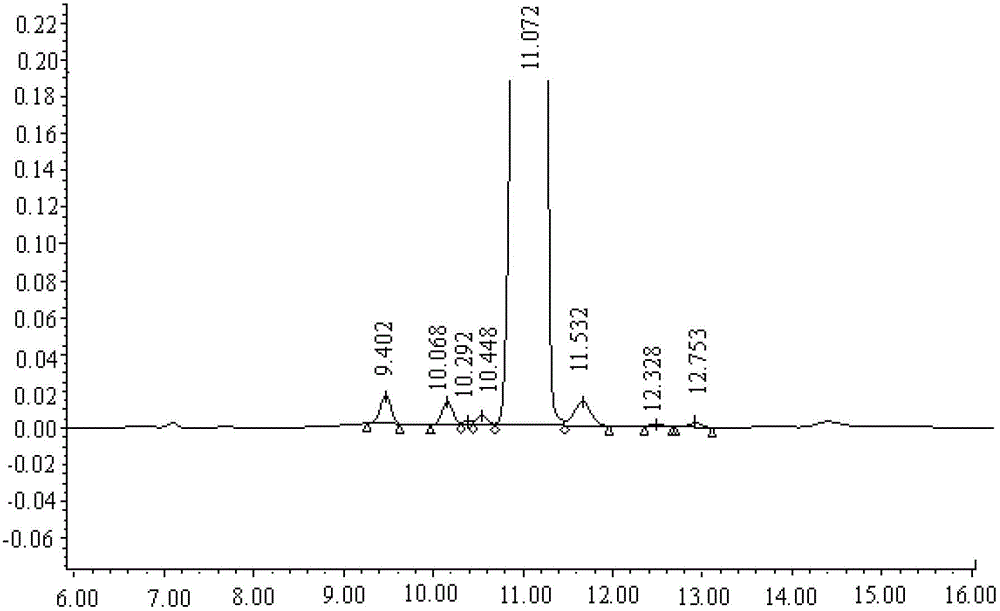
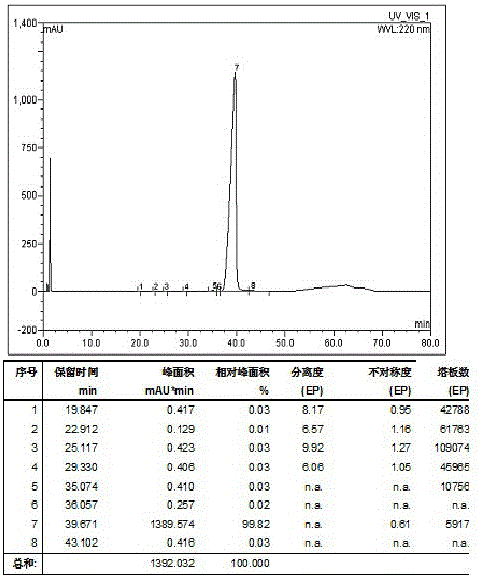
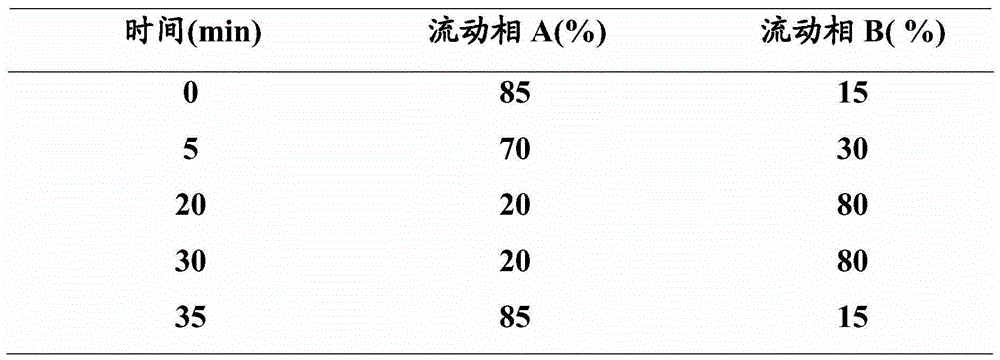
Ultra-high performance liquid chromatogram detection method for terlipressin and impurities thereof
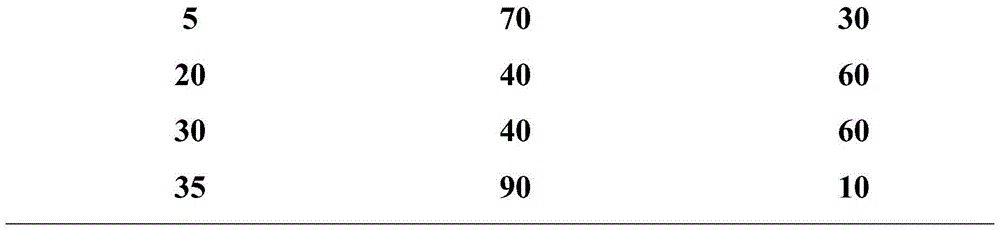
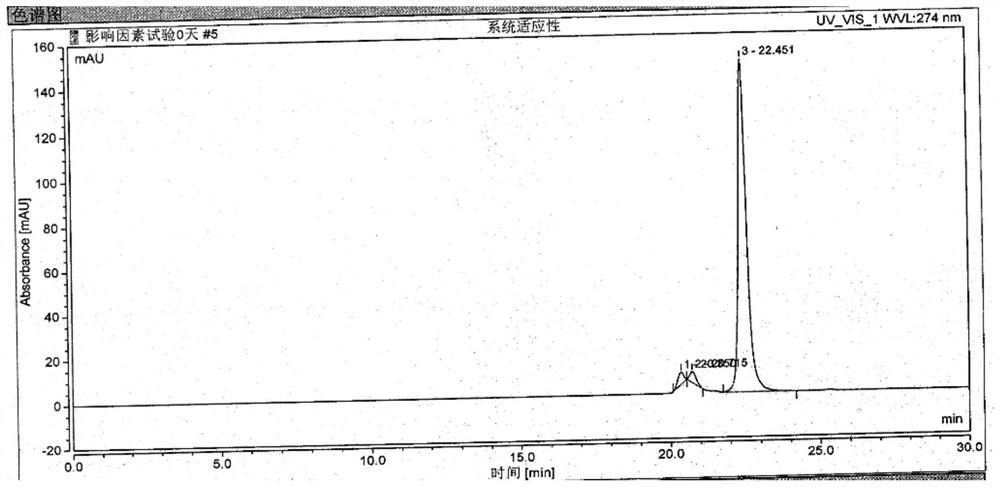
The invention provides an ultra-high performance liquid chromatogram detection method for segregation analysis of terlipressin and impurities thereof. The method comprises the step of and carrying out gradient elution on the terlipressin and the impurities thereof by taking a mobile phase A and a mobile phase B which are prepared from a buffer solution and an organic solvent in different proportions as moving phases in an ultra-high performance liquid chromatograph, thereby realizing rapid segregation analysis of the terlipressin and the impurities thereof.
Owner:HYBIO PHARMA
Preparation method of terlipressin through combination of solid and liquid
InactiveCN105418736AHigh purityReduce the difficulty of synthesisOxytocins/vasopressinsPeptide preparation methodsCyclic peptideCrystallography
The invention relates to the field of polypeptide synthesis, and specifically relates to a preparation method of terlipressin through combination of solid and liquid. According to the preparation method, Boc-Gly-OH and glycylglycine are subjected to liquid phase synthesis so as to obtain a peptide fragment (Boc-Gly-Gly-Gly-OH); at the same time, solid phase synthesis is performed to obtain a cyclic peptide segment (H-c[Cys-Tyr(OtBu)-Phe-Gln(Trt)-Asn(Trt)-Cys]-Pro-Lys(Boc)-Gly-amino resin); finally the peptide fragment and cyclic peptide segment are coupled to prepare terlipressin resin; and then the terlipressin resin is cracked, purified, and freeze-dried to obtain terlipressin. The provided technology reduces the generation of impurity peptides, improves the purity and yield of coarse peptide, simplifies the operation steps, further reduces the production cost, and can be easily applied to large scale enlarged production.
Owner:JINAN KANGHE MEDICAL TECH
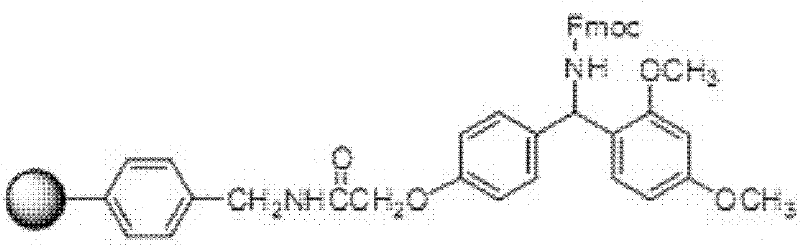
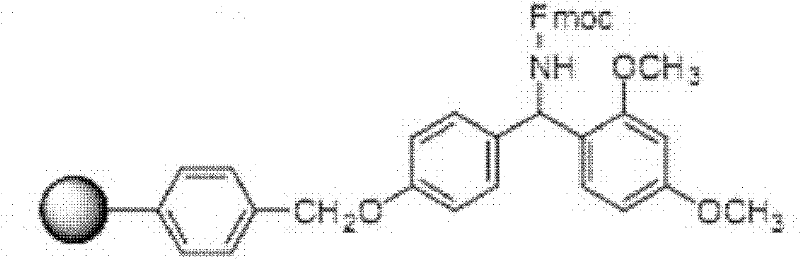
Solid phase polypeptide synthesis preparation method for terlipressin
ActiveCN1865282AReduce usageSimple processOxytocins/vasopressinsPeptide preparation methodsIndustrial constructionRink amide resin
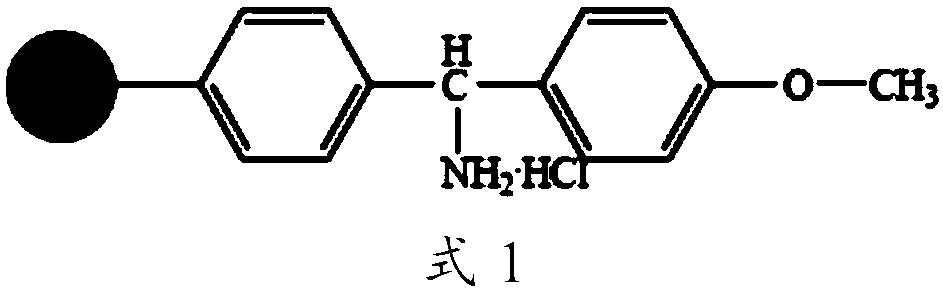
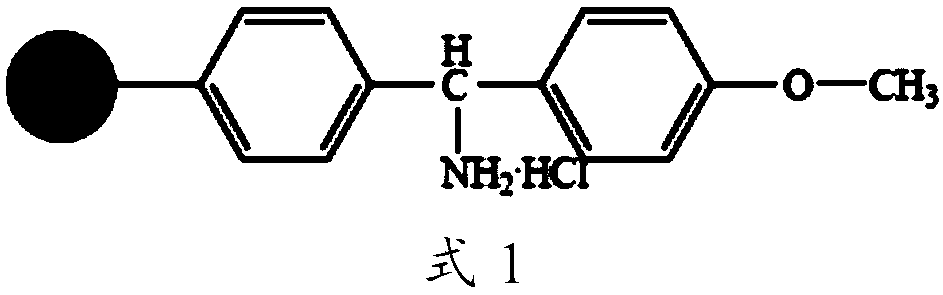
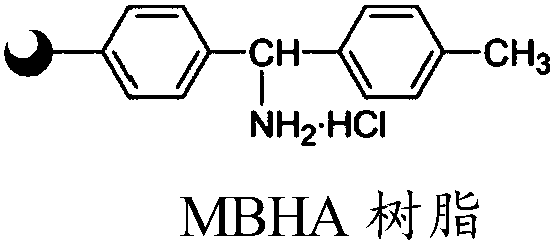
The invention discloses a Telis-vasophysin preparing method of solid-phase polypeptide, which comprises the following steps: adopting Rink Amide resin (concluding Rink Amide MBHA resin, Rink AmideAM resin) as original material, Fmoc protective amino acid as monomer, TBTU or HBTU-to-HOBt as condensing agent to connect amino sequently; using Boc-Gly-OH for the last peptide chain; adding peptide cutting agent to cut peptide; adding ether to deposit to obtain crude product; adding alkali material to oxidate at 7.5-10.0 pH value to generate oxide crude product; proceeding separation and purifying through C18 (or C8) pillar to produce object product. The method possesses low manufacturing cost, simple technology, high obtaining rate and low environmental pollution, which is convenient to do industrial construction.
Owner:SHANGHAI SOHO YIMING PHARMA
Method for preparing terlipressin by virtue of fragment condensation
ActiveCN104371008AHigh purityHigh yieldOxytocins/vasopressinsPeptide preparation methodsPeptide fragmentProtecting group
The invention discloses a fragment condensation method of terlipressin. The method comprises the following steps: performing solid-phase preparation of full-protection first peptide fragment sequence resin of terlipressin, performing cyclization to form disulfide bonds, and then cracking down the cyclized full-protection first peptide fragment sequence from the resin; performing condensation on the cyclized full-protection first peptide fragment sequence and H-Gly-NH2 in a liquid phase to obtain a cyclized full-protection second peptide fragment sequence; performing liquid-phase or solid-phase preparation of a third peptide fragment sequence of the terlipressin; and performing condensation between the cyclized full-protection second peptide fragment sequence after deamination end protection and the third peptide fragment sequence, then removing all protective groups to obtain a terlipressin crude product, and performing purification and salt conversion to obtain a product namely terlipressin acetate, wherein the first peptide fragment sequence is the amino acid of the 4-11th sites in the terlipressin sequence, the second peptide fragment sequence is the amino acid of the 4-12th sites in the terlipressin sequence, and the third peptide fragment sequence is the amino acid of the 1-3rd sites in the terlipressin sequence.
Owner:海南建邦制药科技有限公司
Method for preparing terlipressin
InactiveCN105367627AReduce generationLow costOxytocins/vasopressinsPeptide preparation methodsGlycineBiochemical engineering
The invention relates to a method for preparing terlipressin, and belongs to the technical field of polypeptide synthesis. The method comprises the steps that terlipressin peptide resin is synthesized through a fragment condensation method, 3-12 fragments are synthesized through a solid phase method, 1-2 fragments are connected to 3-12 solid phase amino-acid resin, full-peptide resin is obtained, and the terlipressin is obtained after cracking and cyclizing. Due to the fact that Boc-Gly-Cly-OH is adopted as a raw material, lack of glycine impurities is reduced, the operation step of removing glycine protection groups at an N terminal is omitted, product purity is improved, and the total cost is obviously reduced.
Owner:ANHUI UNIVERSITY OF TECHNOLOGY AND SCIENCE
Solid phase polypeptide synthesis preparation method for terlipressin
ActiveCN1865282BReduce usageSimple processOxytocins/vasopressinsPeptide preparation methodsIndustrial constructionRink amide resin
The invention discloses a Telis-vasophysin preparing method of solid-phase polypeptide, which comprises the following steps: adopting Rink Amide resin (concluding Rink Amide MBHA resin, Rink AmideAM resin) as original material, Fmoc protective amino acid as monomer, TBTU or HBTU-to-HOBt as condensing agent to connect amino sequently; using Boc-Gly-OH for the last peptide chain; adding peptide cutting agent to cut peptide; adding ether to deposit to obtain crude product; adding alkali material to oxidate at 7.5-10.0 pH value to generate oxide crude product; proceeding separation and purifying through C18 (or C8) pillar to produce object product. The method possesses low manufacturing cost, simple technology, high obtaining rate and low environmental pollution, which is convenient to do industrial construction.
Owner:SHANGHAI SOHO YIMING PHARMA
Related substance analysis method for terlipressin for injection
ActiveCN105301156AImprove detection abilityEfficient analysisComponent separationSolventAnalysis method
The invention belongs to the technical field of medicines, and particularly relates to a related substance analysis method for terlipressin for injection. The content detection method for the terlipressin for injection includes the following steps: (Step 1) preparation of test solvent: the terlipressin for injection is prepared into the test solvent; (Step 2) preparation of self-control solvent: the test solvent is diluted, so that the self-control solvent is prepared; (Step 3) related substance content calculation: the control solvent and the test solvent are respectively injected into high-performance liquid chromatographs, so that chromatograms are obtained, and the related substance content in the test solvent is obtained by calculation.
Owner:TIANJIN CHASE SUN PHARM CO LTD
Treatment of ascites
A method for treating ascites patients by administering the peptide drug terlipressin by continuous infusion. The patients include those whose ascites condition has not progressed to hepatorenal syndrome (HRS). Administration may be accomplished with a continuous infusion pump.
Owner:BIOVIE INC
Terlipressinlipidosome and preparation method thereof
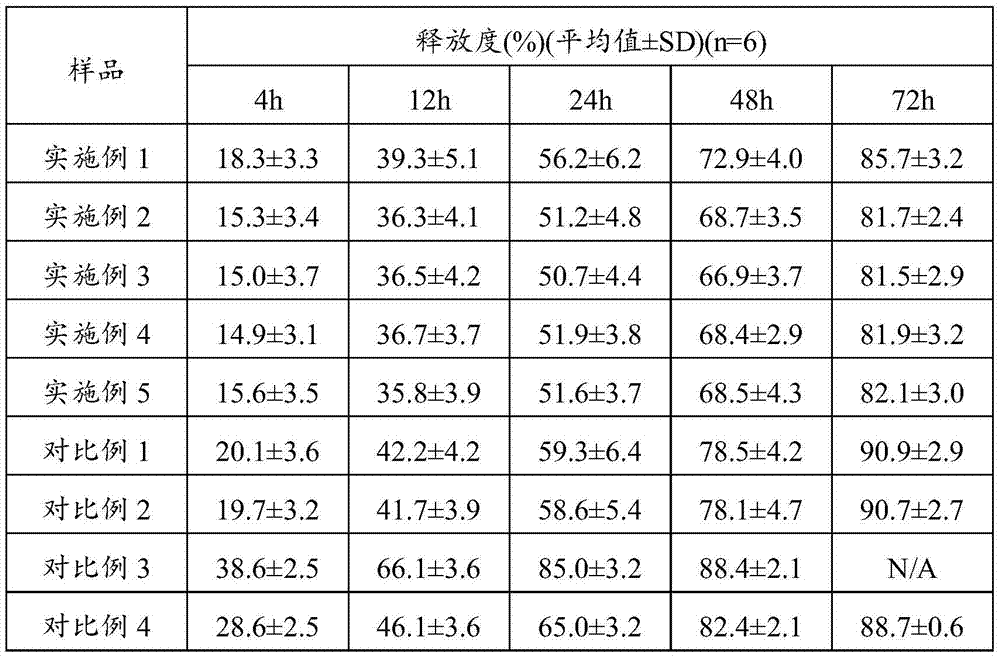
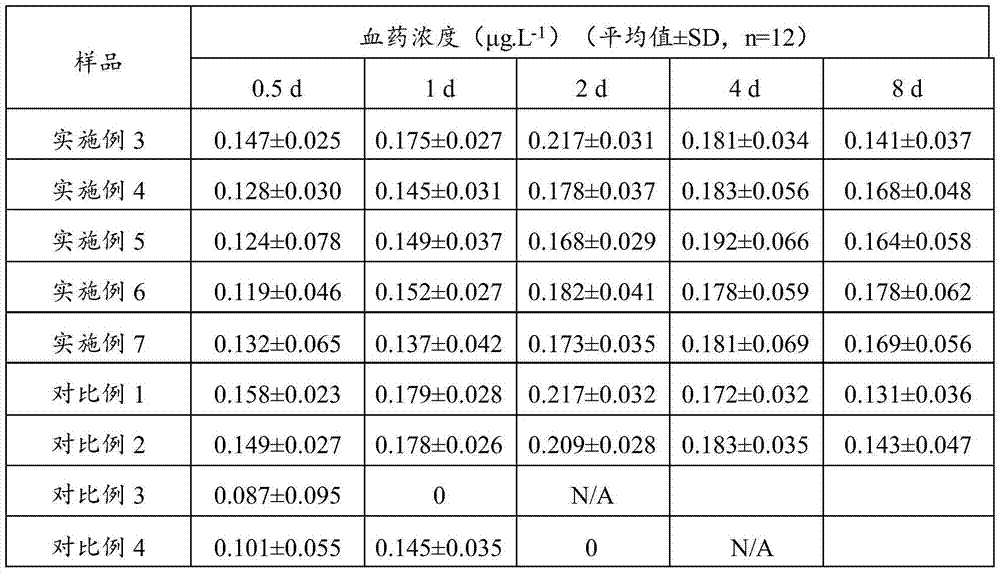
InactiveCN106924715ASolve the shortcoming of short acting timeGood sustained release effectPeptide/protein ingredientsDigestive systemMedicinePlasma concentration
The invention relates to the field of medicines, in particular to terlipressinlipidosome and a preparation method thereof. The invention provides the terlipressinlipidosome, and the pH value of the internal water phase thereof is 7.9 to 8.9. The lipidosomepreparation overcomes the defect that the medicine action time is short. The isoelectric point of the terlipressin is weakly alkaline, the internal pH value of the lipidosome is adjusted to be weakly alkaline, the medicine is deposited under the weakly alkaline condition, and the medicine can be slowly released, so that a bettersustained release effect is achieved. The lipidosome provided by the invention can maintain long action time, the plasma concentration is stable and the occurrence rate of adverse effects is low.
Owner:HYBIO PHARMA
A method for purifying terlipressin
ActiveCN102731625BHigh yieldEasy to operateOxytocins/vasopressinsPeptide preparation methodsTerlipressinImpurity
The present invention relates to the field of polypeptide purification, and particularly to a method for purifying terli. According to the method, a terli crude product is pre-treated, and then purification and salt conversion are performed to obtain the terli with purity more than 99.5%. The method of the present invention has the following advantages that: the operation is simple and easy to perform; the terli prepared by the method of the present invention has characteristics of high purity (the purity can be more than 99.5%, and the maximum single impurity is less than 0.1%) and high yield (the purification yield can be more than 85%, and the total yield can be more than 50%); and the method meets requirements of industrialization, and more than 1000 g of the refined peptide can be obtained in one batch.
Owner:HYBIO PHARMA
Method for detecting high-molecular polymers in terlipressin for injection
ActiveCN110658297AControl contentThe detection method is simpleComponent separationIsocratic elutionFluid phase
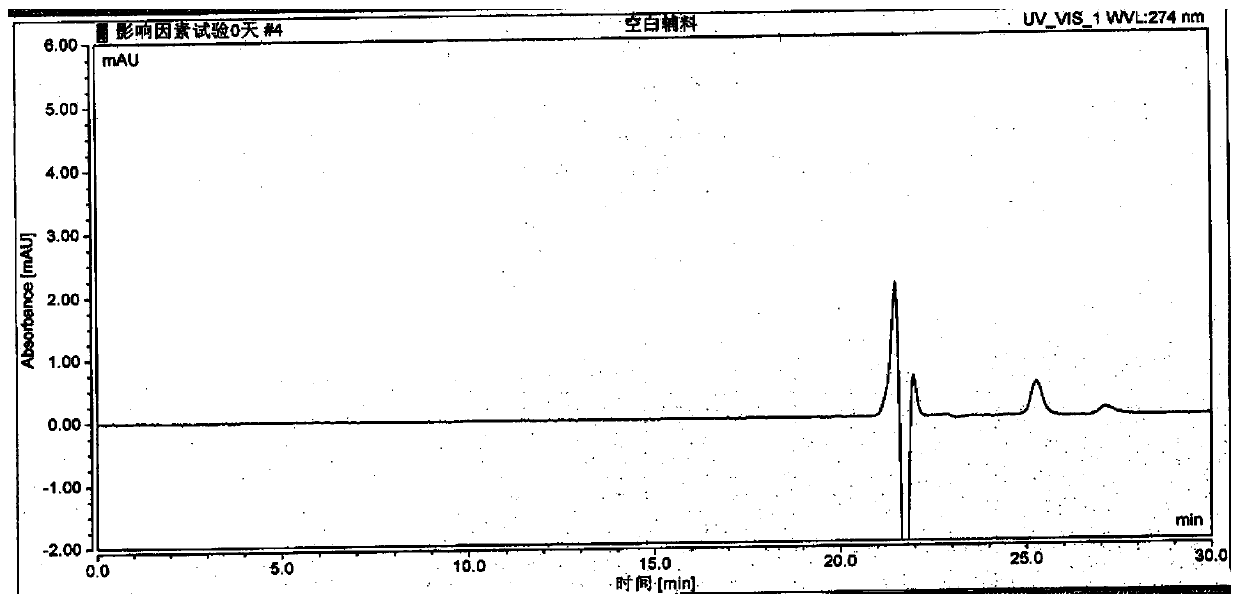
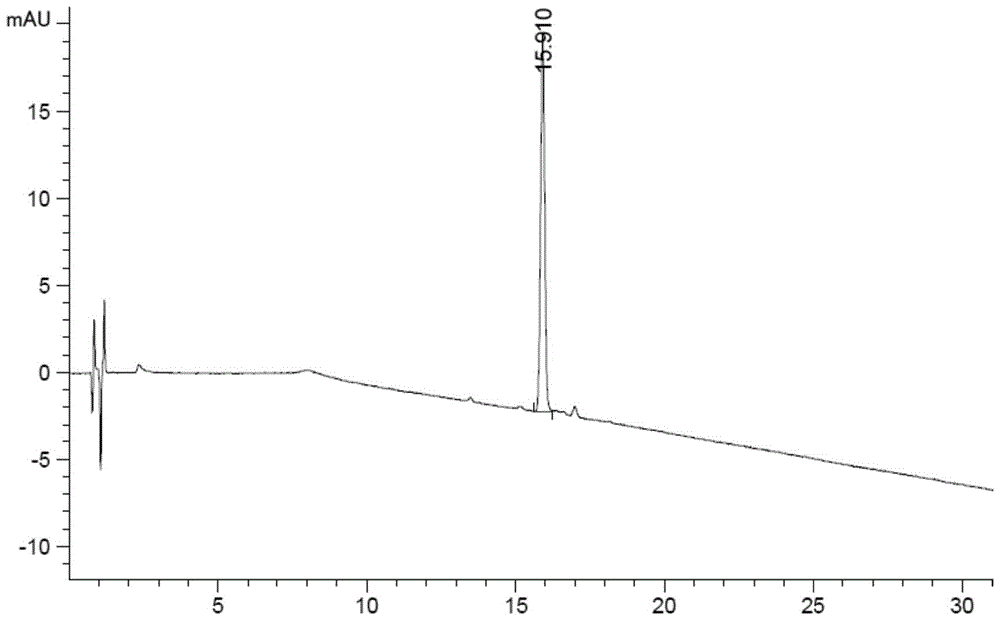
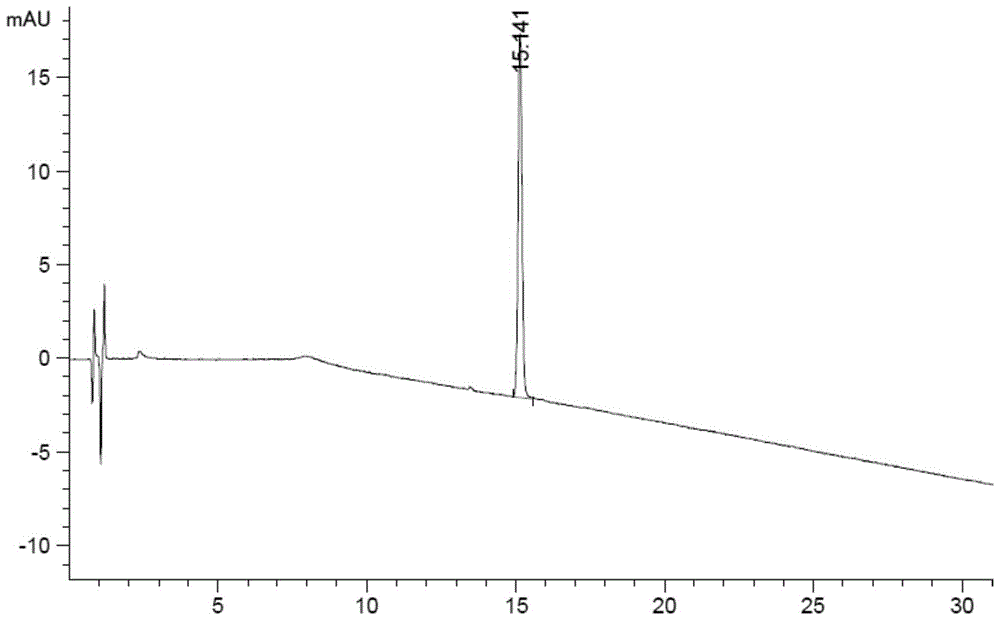
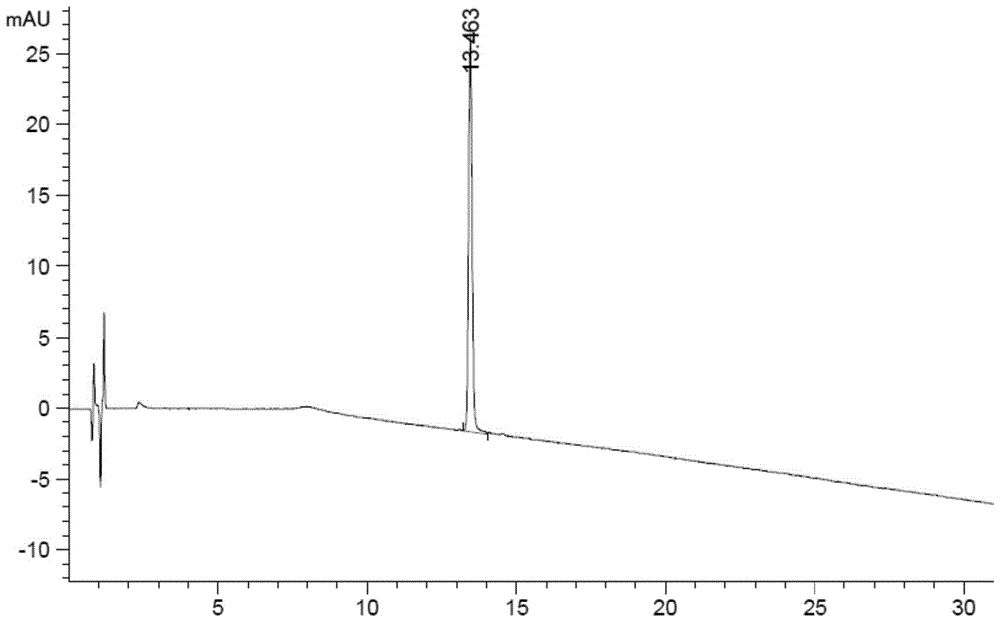
The invention discloses a method for detecting and analyzing high-molecular polymers in terlipressin for injection, and belongs to the technical field of compound detection and analysis. A high performance liquid chromatography is adopted, and chromatographic conditions of the high performance liquid chromatography include the chromatographic column of TSKgel G2000SWXL, the specification of 7.8mm*300mm, the size of 5[mu]m, the mobile phase obtained by preparing a 0.1 mol / L sodium sulfate solution from a 0.1 mol / L phosphate buffer solution, the detection wavelength of 274nm; the flow rate of 0.5 ml / min, the column temperature of 25 DEG C, the sample size of 20 [mu]l, and an isocratic elution mode. The method can be accurately detect the high-molecular polymer in the terlipressin for injection, the content of the high-molecular polymer in a product can be effectively controlled, and the detection method also has the advantages of being simple and highly operable.
Owner:CHINA MEHECO SANYANG PHARMA CO LTD
A method for analyzing related substances of terlipressin for injection
ActiveCN105301156BHigh column efficiencyImprove control standardsComponent separationSolventAnalysis method
The invention belongs to the technical field of medicines, and particularly relates to a related substance analysis method for terlipressin for injection. The content detection method for the terlipressin for injection includes the following steps: (Step 1) preparation of test solvent: the terlipressin for injection is prepared into the test solvent; (Step 2) preparation of self-control solvent: the test solvent is diluted, so that the self-control solvent is prepared; (Step 3) related substance content calculation: the control solvent and the test solvent are respectively injected into high-performance liquid chromatographs, so that chromatograms are obtained, and the related substance content in the test solvent is obtained by calculation.
Owner:TIANJIN CHASE SUN PHARM CO LTD
Terlipressin synthesis method
ActiveCN107778353AHigh yieldLow costOxytocins/vasopressinsPeptide preparation methodsSynthesis methodsCombinatorial chemistry
The invention relates to the field of synthesis of medicines and discloses a terlipressin synthesis method. A brand new MOBHA resin is used to synthesize the terlipressin, the time for the cyclizationin the molecule is adjusted, the cost is lower in comparison with the exiting Rink Amide series resin, the whole yield of an obtained pure product is remarkably increased, and the whole method is simple and easy to operate, is mild in conditions and is suitable for industrial production.
Owner:CHENGDU SHENGNUO BIOPHARM
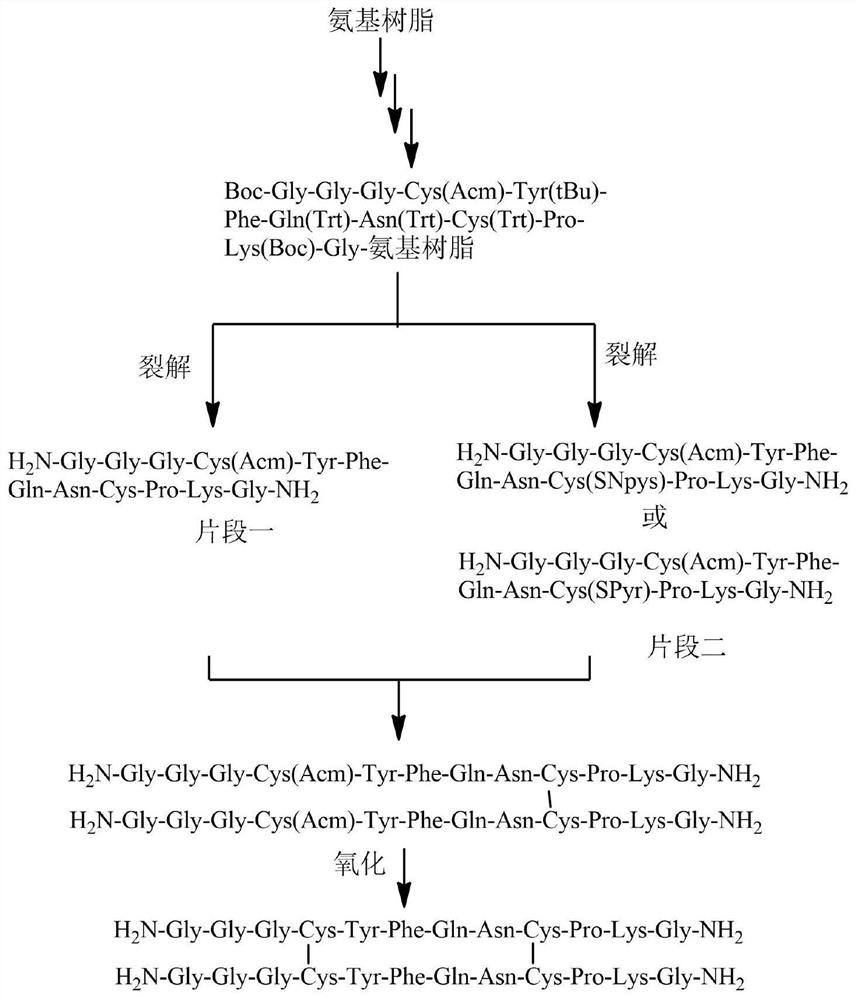
Preparation method of terlipressin impurity Q
PendingCN114685613AOxytocins/vasopressinsPeptide preparation methodsCrystallographyDisulfide bonding
The invention relates to the technical field of polypeptide synthesis, in particular to a preparation method of a terlipressin impurity Q. The preparation method comprises the following steps: firstly synthesizing a polypeptide fragment I and a polypeptide fragment II which are good in stability, then carrying out disulfide bond exchange on SH of a fourth Cys residue at the C end of the polypeptide fragment I and SNpys or SPyr of the fourth Cys residue at the C end of the polypeptide fragment II through condensation reaction to form a first pair of disulfide bonds, and carrying out iodine oxidation reaction to form a second pair of disulfide bonds while removing an Acm protecting group of the Cys. Experiments show that the terlipressin impurity Q obtained by the preparation method is high in purity and few in impurity, and the purity of a crude product can reach 75% or above.
Owner:HYBIO PHARMA
Method for synthesizing terlipressin by solid-phase oxidization and cyclization
ActiveCN101693738BEasy to operatePost-processing is simpleOxytocins/vasopressinsPeptide preparation methodsFreeze-dryingTerlipressin
The invention discloses a method for synthesizing terlipressin by solid-phase oxidization and cyclization. The method comprises the following steps: (1) obtaining Fmoc-Gly-amino resins by using Fmoc-Gly-OH and amino resins; (2) coupling the Fmoc-Gly-amino resins one by one to obtain linear terlipressin-amino resins; (3) synthesizing the linear terlipressin-amino resins into terlipressin-amino resins by adopting iodine solid-phase oxidized cyclization; (4) cracking and cutting the terlipressin-amino resins to obtain raw peptides of the terlipressin; and (5) carrying out purification, salt conversion and freeze-drying on the raw peptides to obtain terlipressin acetate. The invention has the key innovations that solid-phase oxidization reduces a large amount of waste liquid brought by liquid-phase oxidization, accelerates the reaction time, belongs to process greening reformation and greatly improves the total yield.
Owner:HYBIO PHARMA
A method for purifying terlipressin
The invention relates to a purifying method for terlipressin. The method is characterized by comprising the following steps: step 1, dissolving a crude terlipressin product with water and adjusting a pH value to 3 to 4; step 2, flushing a C18 reversed-phase filled column by using an ion pair mobile phase A containing surfactant; step 3, loading a solution obtained in the step 1 into a C18 reversed-phase filling material; step 4, carrying out gradient procedures, wherein the initial-state mobile phase B of an elution gradient is 5% which is maintained for 5 min, then the proportion of the mobile phase B is increased to 30% within 60 min, and a part of target peak elution fraction is collected; and step 5, carrying out nanofiltration membrane desalination and then freeze drying so as to obtain pure terlipressin.
Owner:ADLAI NORTYE BIOPHARMA CO LTD
Application of terlipressin in preparation of drugs for treating dynamic intestinal obstruction
ActiveCN112704727ARemissionGood curative effectAntibacterial agentsOrganic active ingredientsIntestinal ObstructionsHepatic disorders
The invention relates to application of terlipressin in preparation of drugs for treating dynamic intestinal obstruction. The dynamic intestinal obstruction is complicated by middle- / late-stage hepatic diseases and can also be complicated by hepatic cirrhosis and primary peritonitis. According to the application disclosed by the invention, the technical scheme can support and supplement the existing treatment schemes to some extent and has an important clinical significance in treatment on sufferers suffering from complicated hepatic-cirrhosis-complicated dynamic intestinal obstruction.
Owner:THE SECOND AFFILIATED HOSPITAL OF CHONGQING MEDICAL UNIV
A kind of preparation method of terlipressin and pharmaceutical composition thereof
ActiveCN108659104BEasy to operateImprove stabilityOxytocins/vasopressinsPowder deliveryFluid phaseEngineering
The invention belongs to the technical field of biological medicines, and in particular relates to a preparation method of terlipressin and a pharmaceutical composition containing the terlipressin. The preparation method adopts two different steps to prepare a liquid phase so as to obtain the terlipressin; the method, provided by the invention, for purifying the terlipressin is simple and easy tooperate; the purity of obtained terlipressin reaches 99.7% or above, and the yield can reach 60% or above. A lyophilized powder injection prepared from the composition provided by the invention is good in solubility, stability and resolubility; the lyophilized powder injection prepared from the composition is convenient to use, easy to storage and transport, simple in preparation method, suitablefor industrial production and low in production cost.
Owner:北京市新里程医药科技有限公司
An ultra-high performance liquid chromatography detection method for terlipressin and its impurities
The invention provides an ultra-high performance liquid chromatogram detection method for segregation analysis of terlipressin and impurities thereof. The method comprises the step of and carrying out gradient elution on the terlipressin and the impurities thereof by taking a mobile phase A and a mobile phase B which are prepared from a buffer solution and an organic solvent in different proportions as moving phases in an ultra-high performance liquid chromatograph, thereby realizing rapid segregation analysis of the terlipressin and the impurities thereof.
Owner:HYBIO PHARMA
A kind of detection method of polymer in terlipressin for injection
ActiveCN110658297BControl contentThe detection method is simpleComponent separationPhosphateChromatography column
The invention discloses a method for detecting and analyzing high-molecular polymers in terlipressin for injection, which belongs to the technical field of compound detection and analysis. As follows: Chromatographic column: TSKgel G2000SWXL, size 7.8mm×300mm, 5um; mobile phase: 0.1mol / L sodium sulfate solution prepared with 0.1mol / L phosphate buffer; detection wavelength: 274nm; flow rate: 0.5ml / min; Temperature: 25°C; Injection volume: 20μl; Elution method: Isocratic elution. The invention can accurately detect the content of high molecular polymer in the terlipressin for injection, can effectively control the content of high molecular polymer in the product, and the detection method of the invention has the advantages of simple method and strong operability.
Owner:CHINA MEHECO SANYANG PHARMA CO LTD
Biological sample pretreatment method of vasopressin and analogues thereof
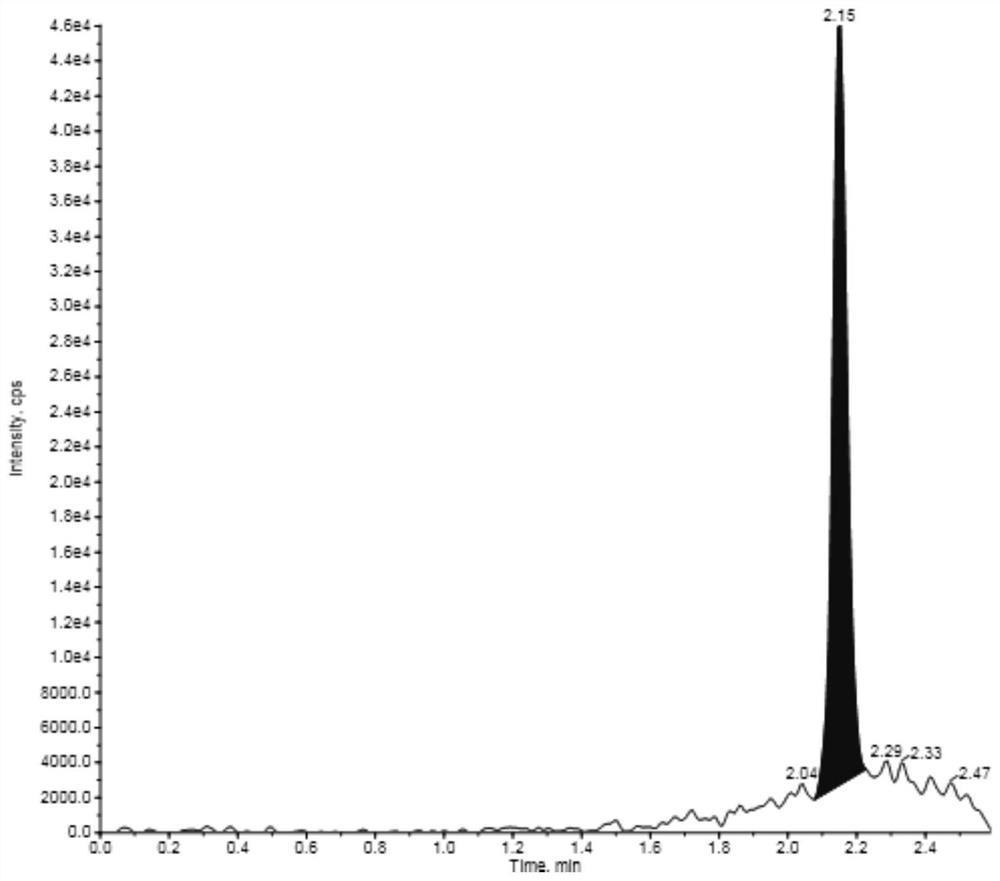
InactiveCN111812238ASolve problems that reduce the accuracy of detection analysisLow costComponent separationAcetic acidMethanol water
The invention provides a biological sample pretreatment method of vasopressin and analogues thereof. The method mainly comprises the following steps of melting a biological sample in an ice bath undera dark condition; vortex blending, sucking the sample into a centrifugal tube and then adding ammonia water into the sample; swirling, adding a terlipressin internal standard, swirling uniformly, activating a 96-well plate, transferring the sample into the 96-well plate, leaching with ammonia water, methanol and acetic acid methanol in sequence, eluting twice with TFA methanol, collecting eluenton a new 96-well plate, blow-drying the eluent, redissolving with a methanol aqueous solution, swirling, introducing a sample and analyzing. The method is high in treatment speed, low in cost, simpleand convenient, easy to popularize and capable of effectively solving a problem that in the detection process of the vasopressin and the analogues of the vasopressin, and chromatographic detection andanalysis accuracy is reduced due to too many impurities.
Owner:苏州旭辉检测有限公司
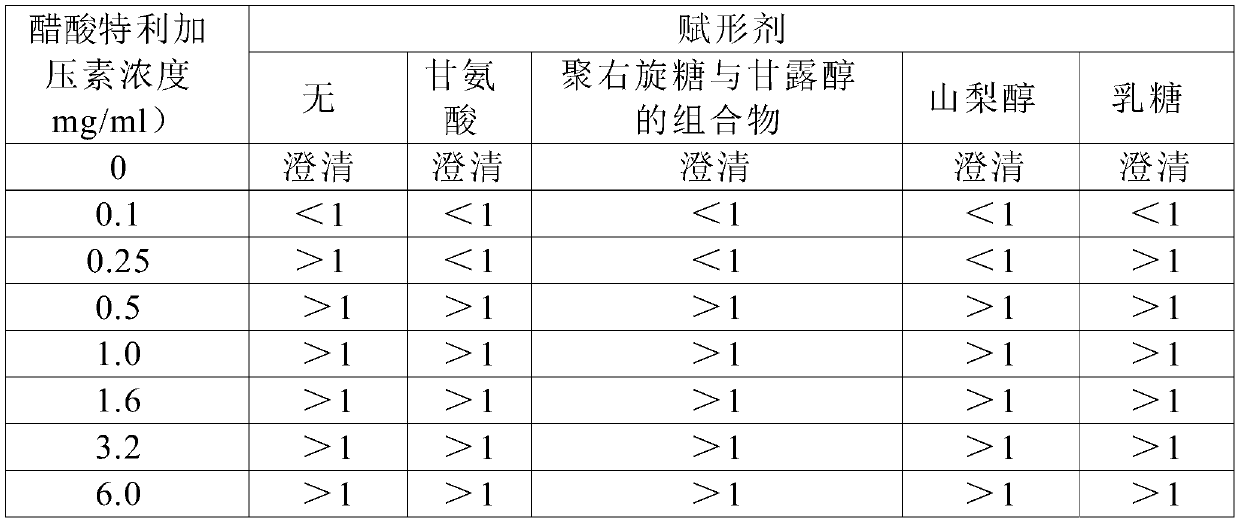
Formulations of terlipressin
PendingUS20220233634A1Infusion syringesInorganic non-active ingredientsVasopressin preparationPharmaceutical Substances
The disclosure provides pharmaceutical compositions comprising terlipressin having increased concentration with long term storage stability.
Owner:BIOVIE INC
Novel composition
PendingUS20220080023A1Peptide/protein ingredientsInorganic non-active ingredientsTonicityAqueous solution
The invention provides inter alia an aqueous solution composition of pH in the range 4.0-6.0 comprising: —terlipressin or a salt thereof; —optionally one or more buffers being substances having at least one ionisable group with a pKa in the range 3.0 to 7.0 and which pKa is within 1 pH unit of the pH of the composition; —optionally an amino acid; and—optionally a tonicity modifier wherein the buffers are present in the composition at a total concentration of 0-5 mM.
Owner:ARECOR LTD
Method for preparing terlipressin by fragment condensation
ActiveCN104371008BHigh purityHigh yieldOxytocins/vasopressinsPeptide preparation methodsPeptide sequenceCombinatorial chemistry
The invention discloses a fragment condensation method of terlipressin. The fully protected first peptide fragment sequence resin of terlipressin is prepared in solid phase, cyclized to form a disulfide bond, and then the cyclized fully protected first peptide is cyclized. The peptide fragment sequence is cleaved from the resin; the cyclized fully protected first peptide fragment sequence is condensed with H-Gly-NH2 in the liquid phase to obtain the cyclized fully protected second peptide fragment sequence; liquid phase or solid phase preparation of terlipress The third peptide fragment sequence of the protein; the cyclized fully protected second peptide fragment sequence is de-protected at the amino terminal and condensed with the third peptide fragment sequence, and then removes all protecting groups to obtain the crude terlipressin, which is purified and converted to salt to obtain the product Terlipressin acetate; the first peptide fragment sequence is the 4-11th amino acid in the terlipressin sequence, the second peptide fragment sequence is the 4-12th amino acid in the terlipressin sequence, the The tripeptide fragment sequence is the 1-3 amino acid in the terlipressin sequence.
Owner:海南建邦制药科技有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com