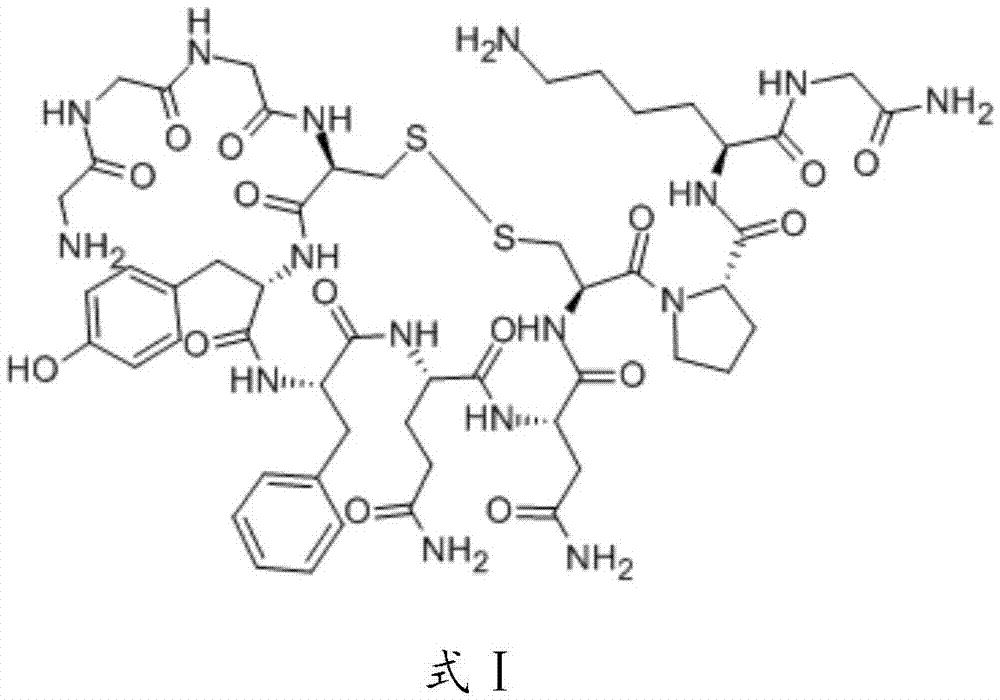
Terlipressinlipidosome and preparation method thereof
A technology of terlipressin and liposomes, which is applied in liposome delivery, pharmaceutical formulations, peptide/protein components, etc., can solve the problem of short half-life, low bioavailability, and short biological half-life of terlipressin, etc. problems, to achieve the effect of low incidence of adverse reactions, stable blood concentration and long action time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
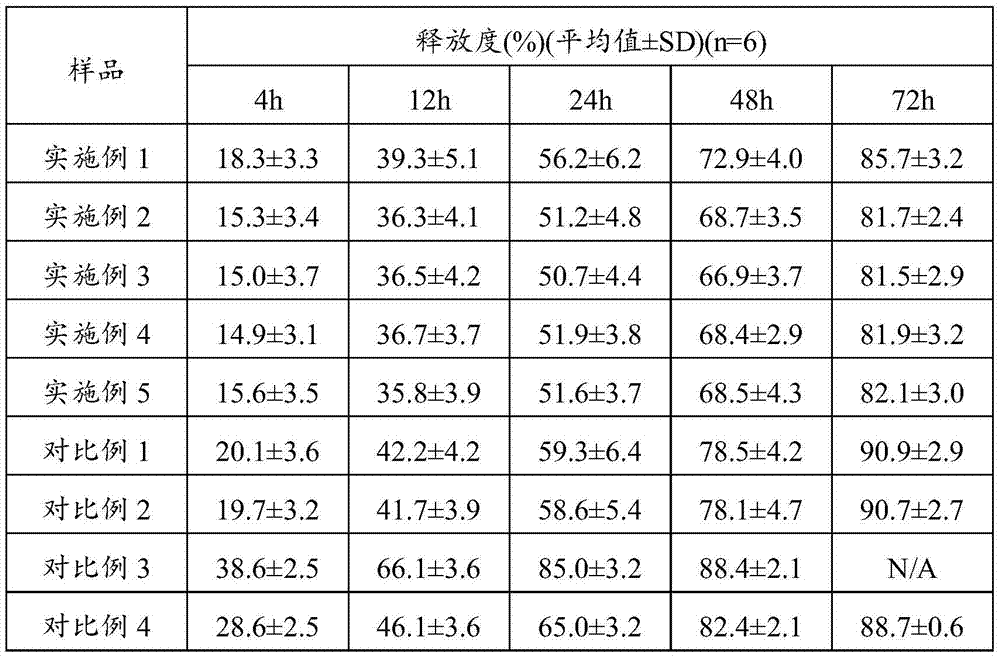
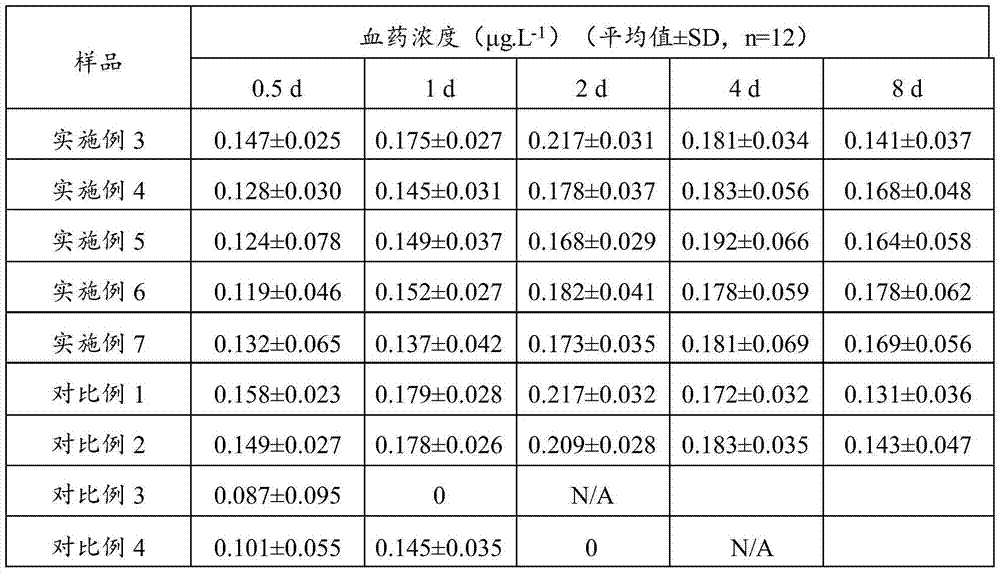
Embodiment 1
[0049] Dissolving 500 mg of the drug terlipressin in 20 mL of the aqueous phase to obtain an aqueous phase containing the active ingredient;
[0050] DSPE-PEG20002g was dissolved in 20mL of chloroform (oil phase), placed in a round bottom flask, and the organic phase was removed by rotary evaporation;
[0051] Add the water phase, prepare the pH between sodium bicarbonate and sodium hydroxide to be between 8.0, vortex to obtain rough liposomes, first homogenize and then pass through a 0.22 μm filter membrane, and use the dialysis membrane method to remove the free water phase, Promptly obtain the liposome solution of medicine.
Embodiment 2
[0053] Dissolving 5 mg of the drug terlipressin in 20 mL of the aqueous phase to obtain an aqueous phase containing the active ingredient;
[0054] 2g of PEG-PE and 1g of cholesterol were dissolved in 20mL of chloroform (oil phase), placed in a round bottom flask, and the organic phase was removed by rotary evaporation;
[0055] Add the water phase, the pH prepared by ammonium sulfate, sodium hydroxide, and sucrose is between 8.4 and 8.5, vortex to obtain rough liposomes, first homogenize and then pass through a 0.22 μm filter membrane, and use the dialysis membrane method to remove free liposomes Aqueous phase, promptly obtains the liposome solution of medicine.
Embodiment 3
[0057] Dissolving 5 mg of the drug terlipressin in 20 mL of the water phase to obtain the water phase;
[0058] Dissolve 2g of DSPE-PEG2000 and 1g of cholesterol in 20mL of chloroform (oil phase), put in a round bottom flask, and remove the organic phase by rotary evaporation;
[0059] Add the water phase, the pH prepared by sodium carbonate and citric acid is between 7.9 and 8.0, vortex to obtain rough liposomes, first homogenize and then pass through a 0.22 μm filter membrane, and use the dialysis membrane method to remove the free water phase. Promptly obtain the liposome solution of medicine.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com