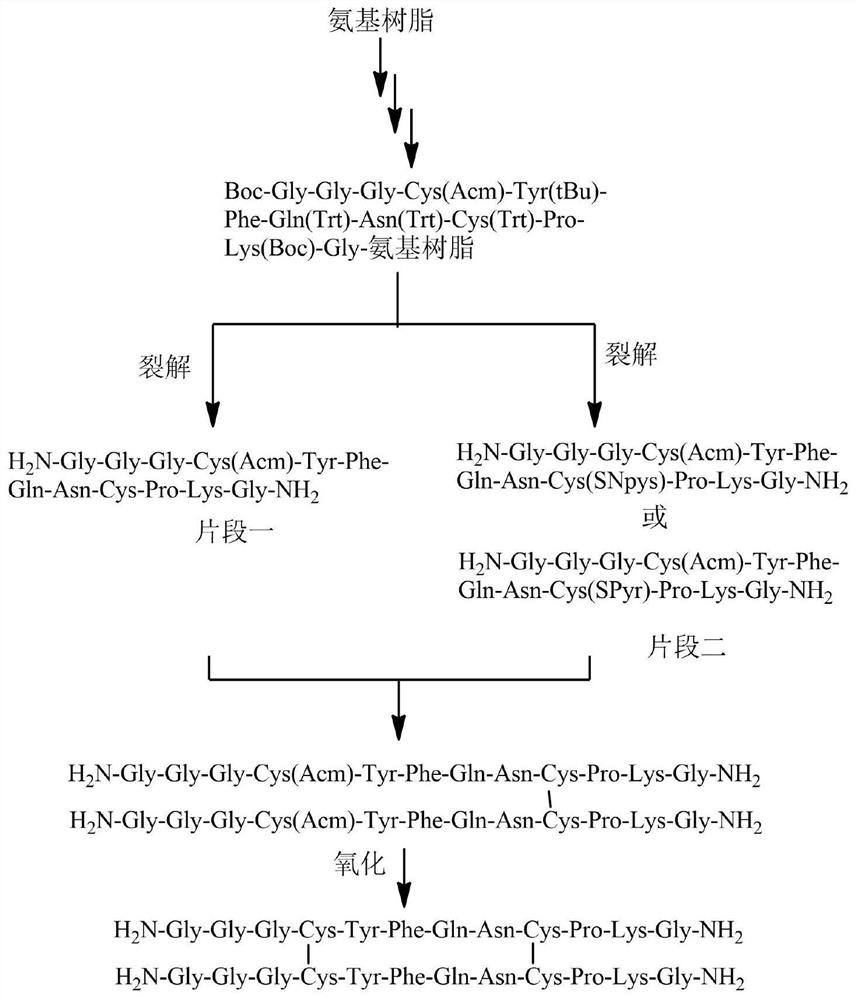
Preparation method of terlipressin impurity Q
A technology of terlipressin and impurities, which is applied in the field of preparation of terlipressin impurity Q, and can solve the problems of not preparing impurity Q, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] Example 1 Synthesis of Polypeptide Fragment One
[0059] Weigh 16.7 g of Rink Amide resin (the substitution degree is 0.60 mmol / g, the scale is 10 mmol), the resin is swollen with DMF for 30 minutes, the resin is drained, and washed twice with DMF. 20% piperidine / DMF solution was added to remove the Fmoc protecting group, the reaction was stirred at room temperature for 10 minutes, the resin was sucked dry, the operation was repeated once, and then washed with DMF for 6 times. In addition, take Fmoc-Gly-OH 8.92g (30mmol), HOBT 4.86g (36mmol) and 60ml DMF, stir to dissolve, add 5.63ml (36mmol) of DIPCDI under ice bath for activation, add it to the resin, react at room temperature for 2 hours, Use the kaiser method to detect whether the reaction is complete. If the resin is colorless and transparent, it means the reaction is complete; if the resin develops color, it means the reaction is not complete, and the above operation needs to be repeated for two shots. This judgm...
Embodiment 2
[0063] Example 2 Synthesis of Polypeptide Fragment II
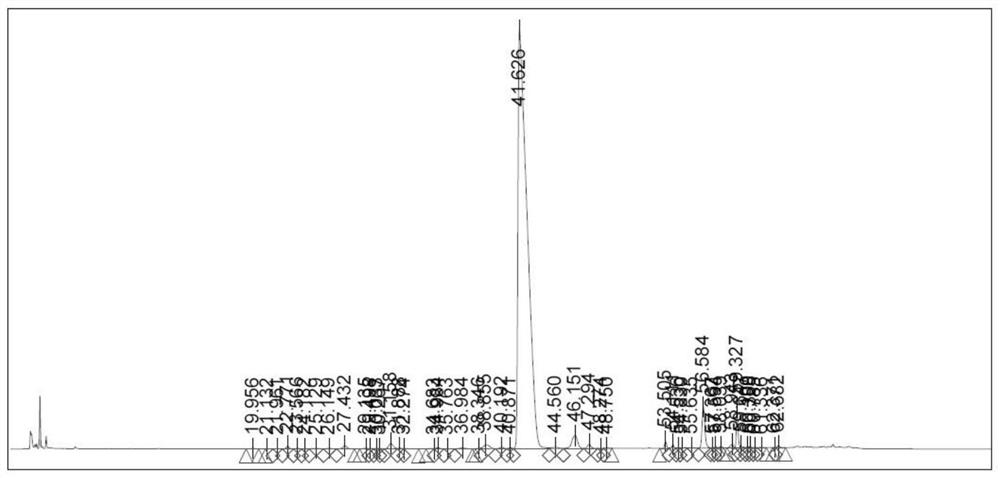
[0064] Take the peptide resin in Example 1 and add it to the lysis solution (TFA:H 10ml / g) 2 (O:DTDP=90:5:5), stirred and reacted at room temperature for 2 hours, filtered, poured the filtrate into frozen anhydrous ether for precipitation, centrifuged, washed three times with anhydrous ether, and vacuum-dried to obtain polypeptide fragment II 16.9g with a purity of 88.05 %.
Embodiment 3
[0065] Example 3 Synthesis of Polypeptide Fragment II
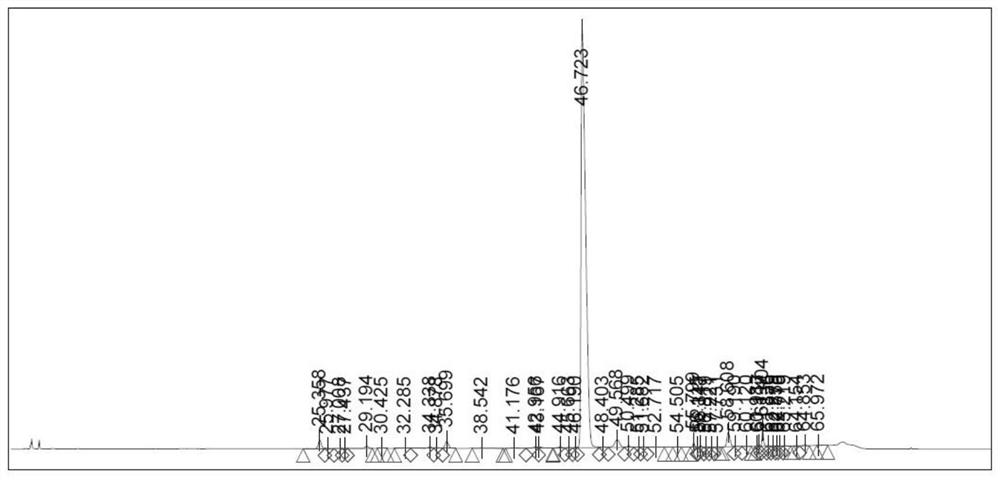
[0066] Take the peptide resin in Example 1 and add it to the lysis solution (TFA:H 10ml / g) 2 O:DTNP=90:5:5), stirred at room temperature for 2 hours, filtered, poured the filtrate into frozen anhydrous ether for precipitation, centrifuged, washed three times with anhydrous ether, and vacuum-dried to obtain polypeptide fragment II 17.4g with a purity of 86.43 %. chromatogram as image 3 The chromatographic data for typical characteristic peaks are shown in Table 3.
[0067] table 3
[0068]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com