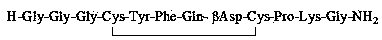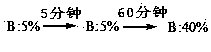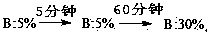Purifying method for terlipressin
A technology of pure terlipressin, which is applied in the field of polypeptide drugs, can solve the problem of low yield of terlipressin finished products, inability to effectively separate terlipressin, and insufficient purity of terlipressin finished products. Advanced problems, to achieve the effect of low cost and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0088] Solid Phase Synthesis of Terlipressin: Synthesis of Terlipressin on a 10 mmol Scale
[0089] (1) Deprotection of peptide resin: 13.89 g of Rink amide AM resin (substitution degree: 0.72 mmol / g) was added to the solid-phase reaction column, washed once with DMF, and the resin was swollen with DCM for 30 min. The deprotection solution composed of 1:4 piperidine and DMF was reacted for 5 min, washed once with DMF, and reacted with a deprotection solution composed of piperidine and DMF with a volume ratio of 1:4 for 10 min, and washed 6 times with DMF.
[0090] (2) Coupling: 8.92 g Fmoc-Gly-OH (30 mmol) and 4.05 g HOBt (30 mmol) were dissolved in the solvent DMF, the ice bath cooling temperature was 0-10 ℃, and 5 mL DIC (30 mmol) was added, After activating for 5 minutes, add it to the reaction column to react with the deprotected resin. After reacting for 2 hours at 25-35 ℃, use the ninhydrin method to detect and judge the reaction end point. If the resin is colorless and ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Mwco | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com