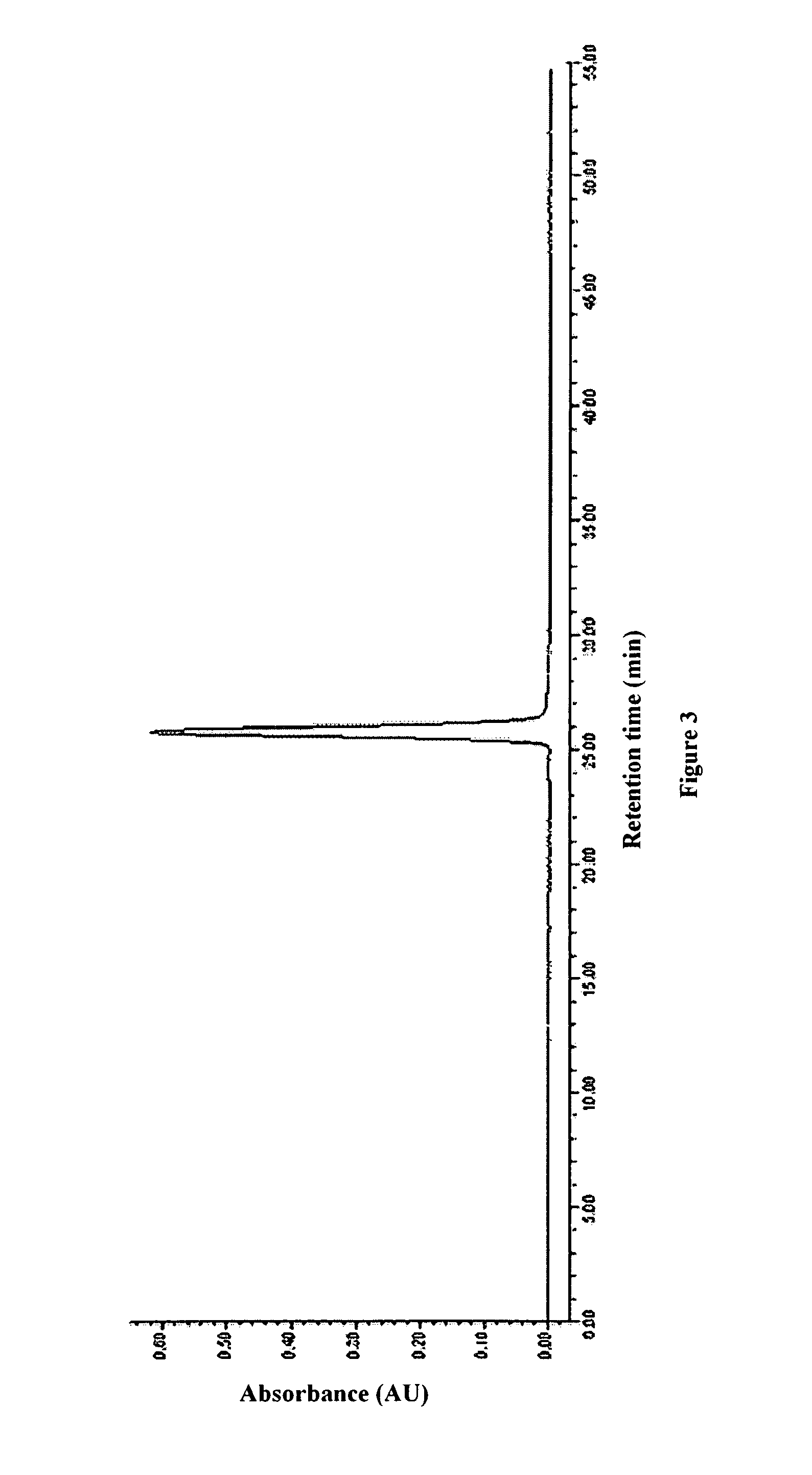
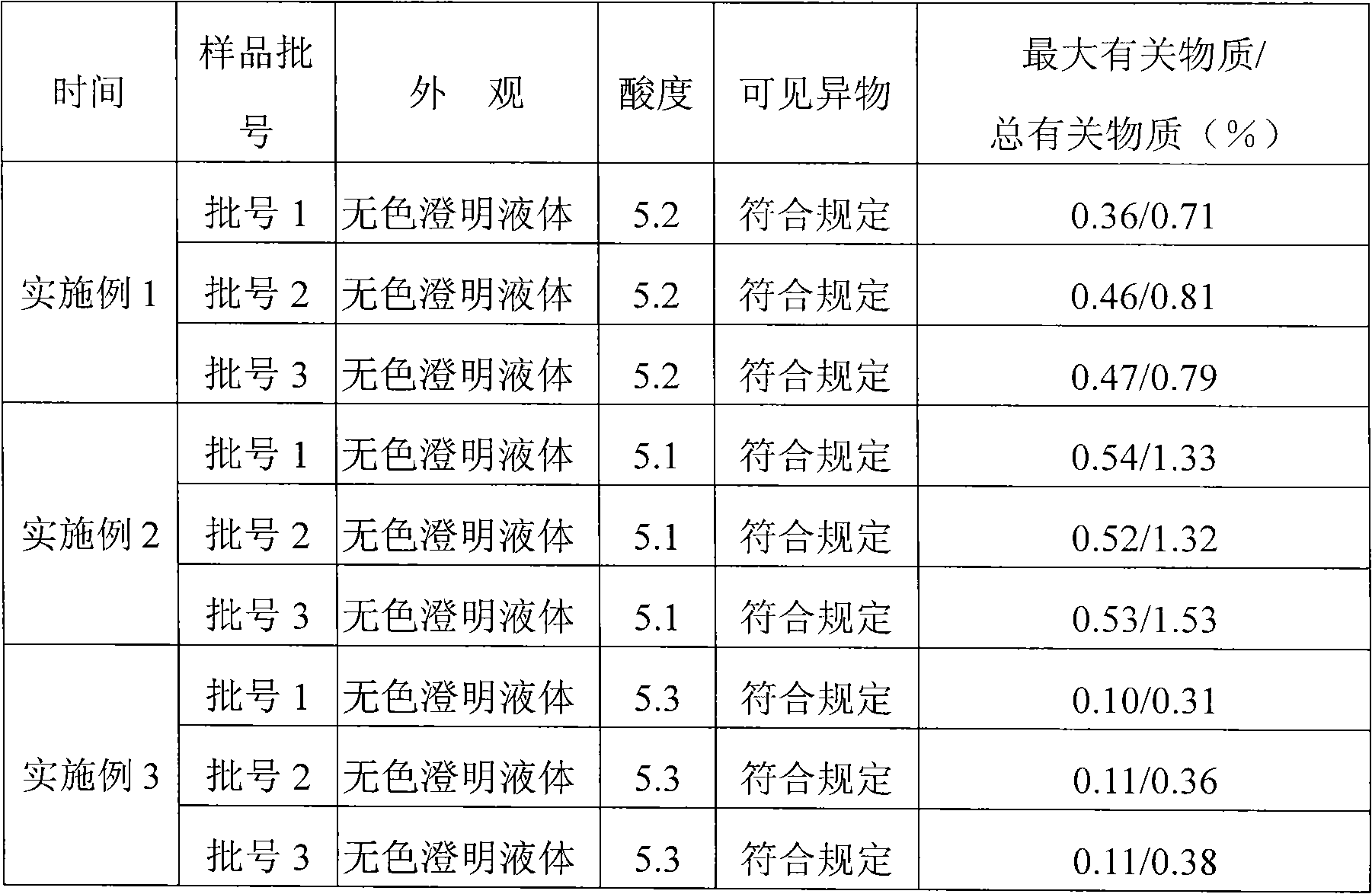
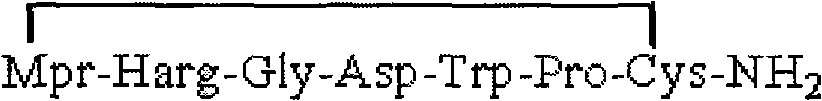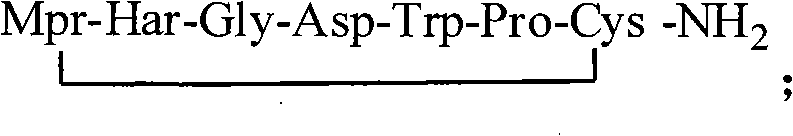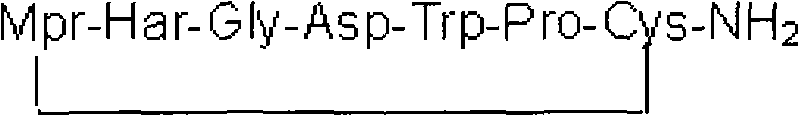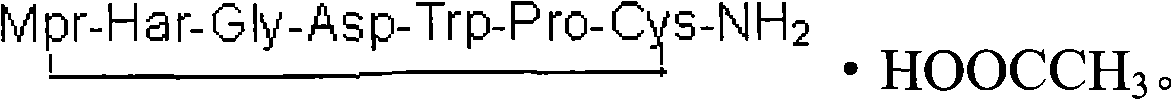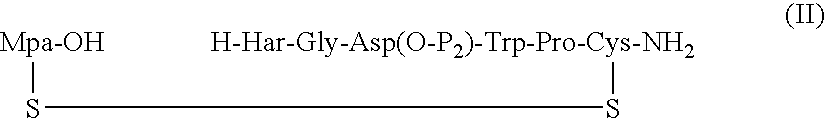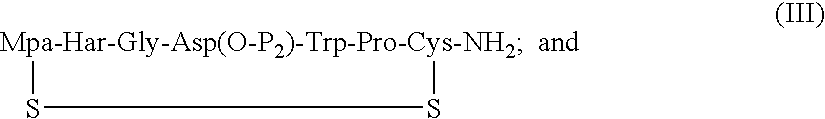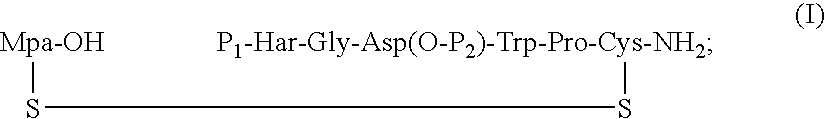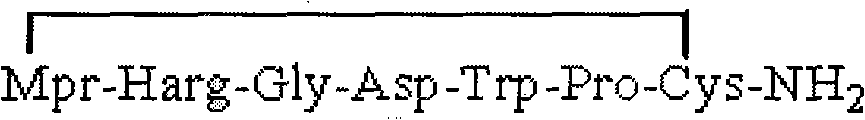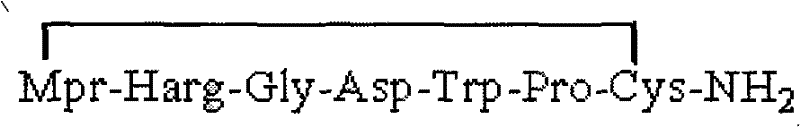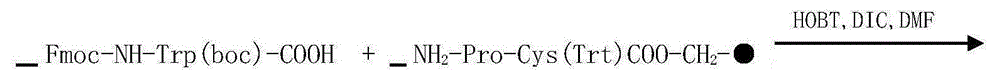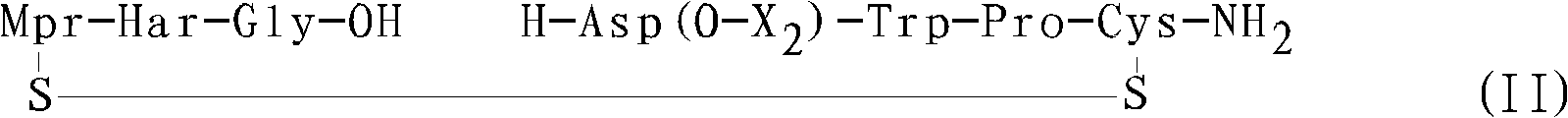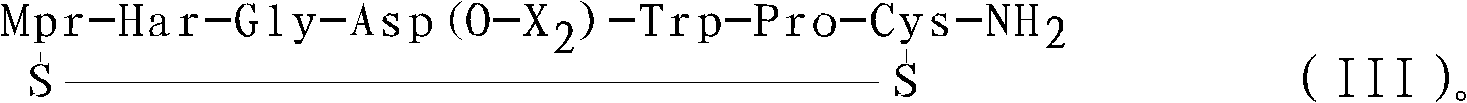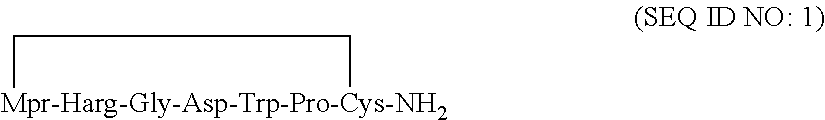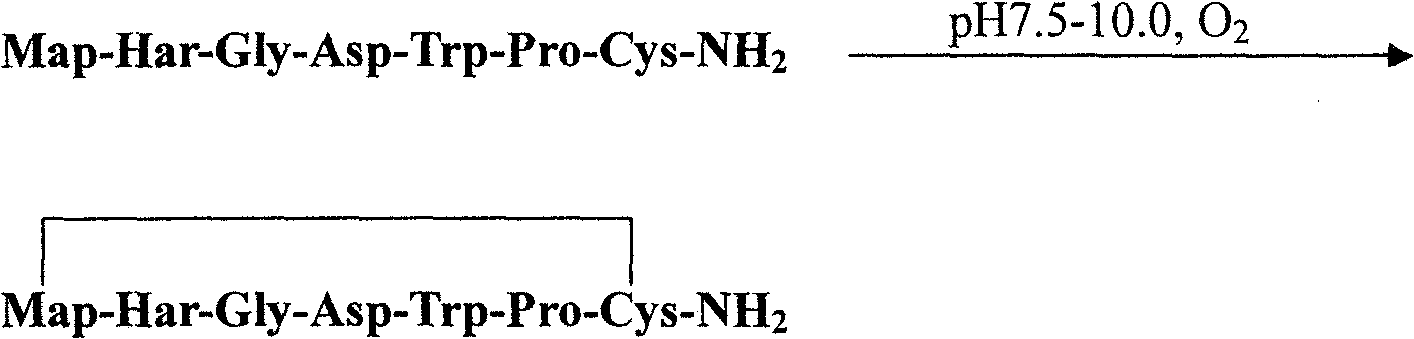Patents
Literature
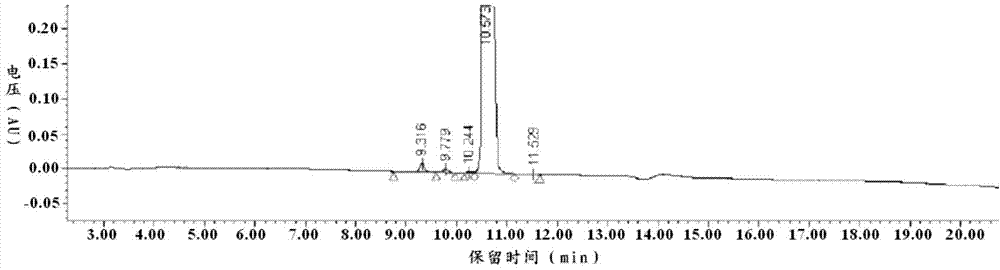
52 results about "Eptifibatide" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
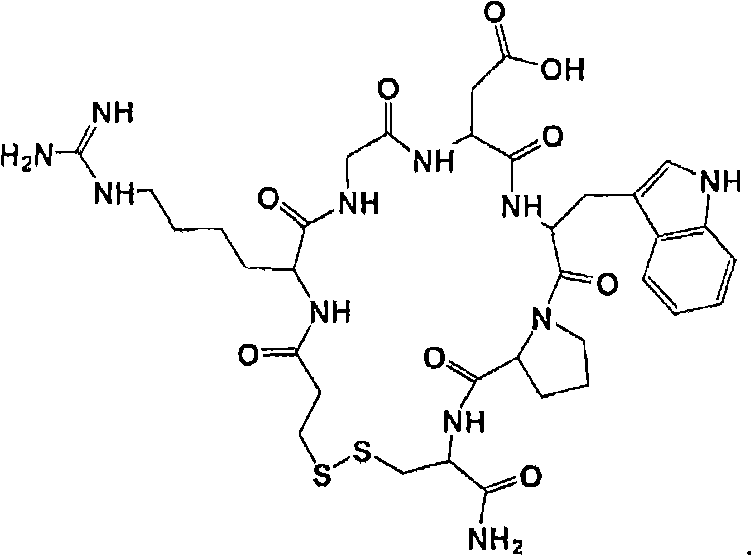
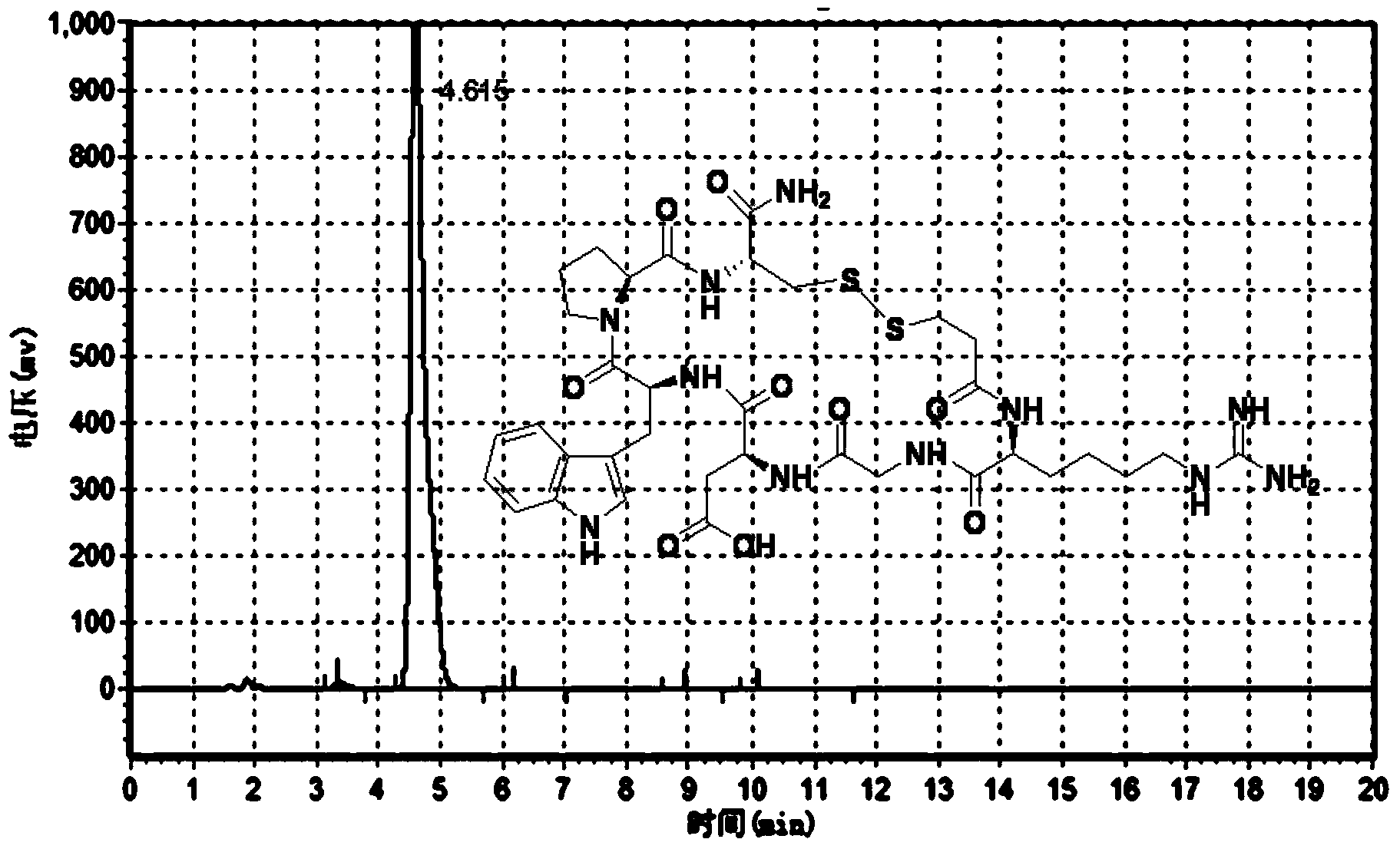
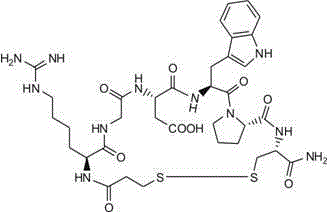
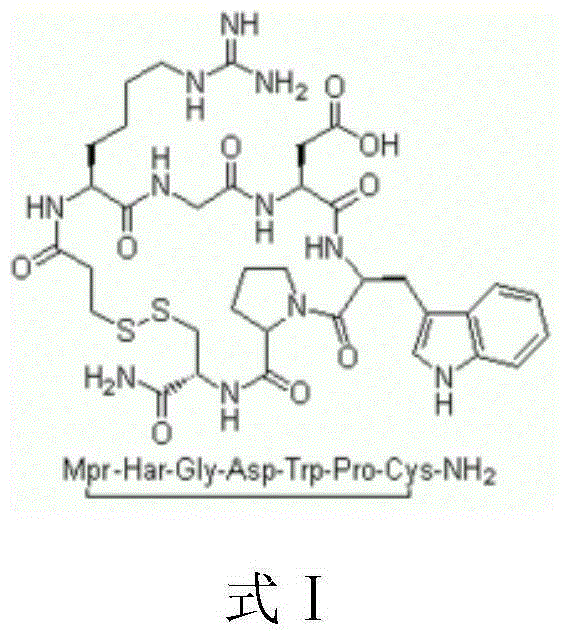
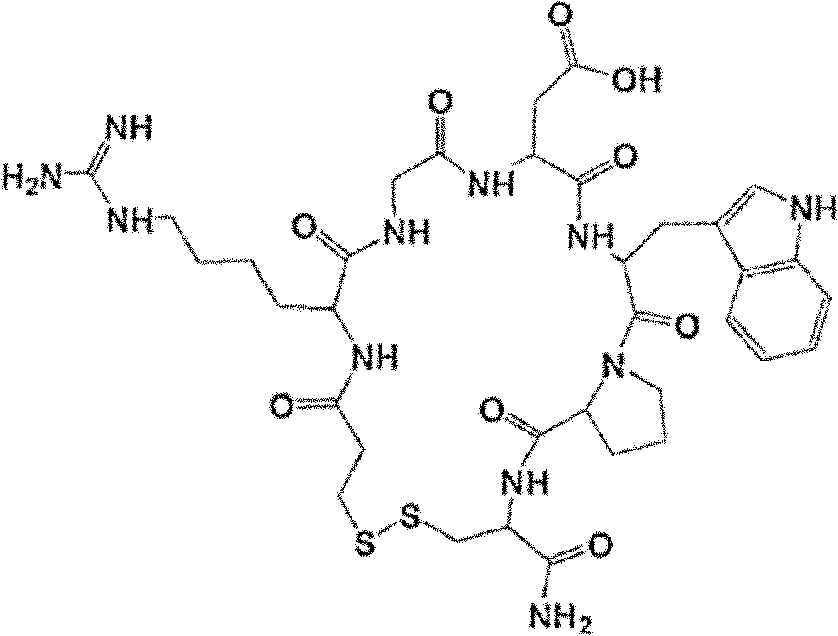
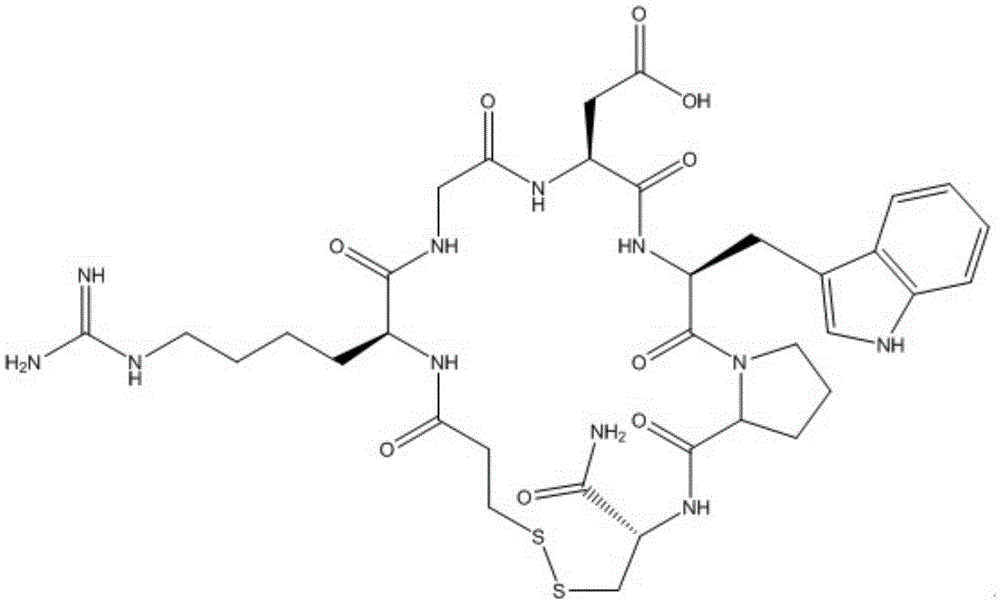
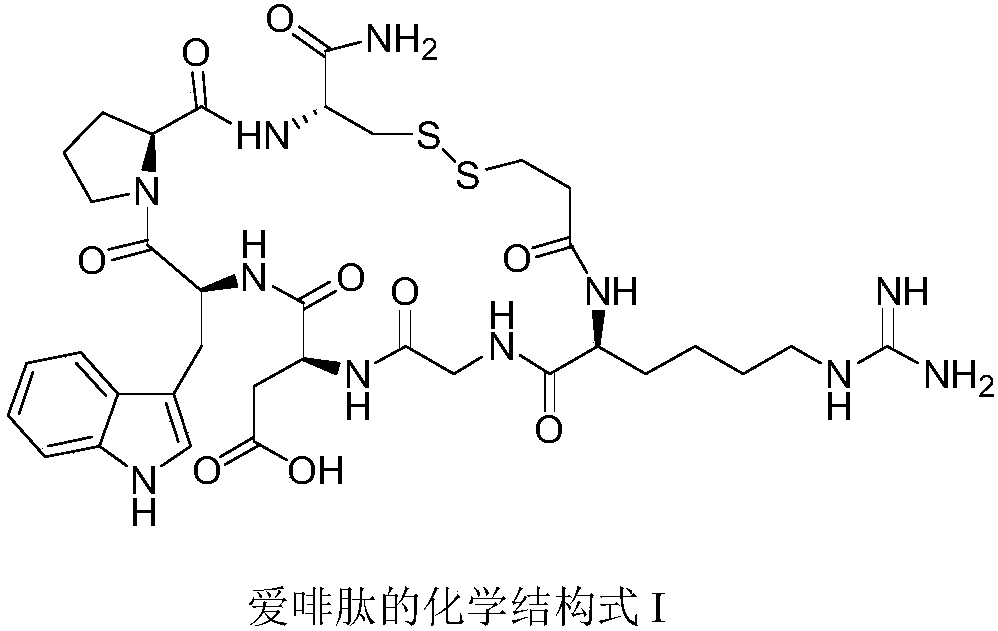
Eptifibatide (Integrilin, Millennium Pharmaceuticals, also co-promoted by Schering-Plough/Essex), is an antiplatelet drug of the glycoprotein IIb/IIIa inhibitor class. Eptifibatide is a cyclic heptapeptide derived from a disintegrin protein (P22827) found in the venom of the southeastern pygmy rattlesnake (Sistrurus miliarius barbouri). It belongs to the class of the arginin-glycin-aspartat-mimetics and reversibly binds to platelets. Eptifibatide has a short half-life. The drug is the third inhibitor of GPIIb/IIIa that has found broad acceptance after the specific antibody abciximab and the non-peptide tirofiban entered the global market.
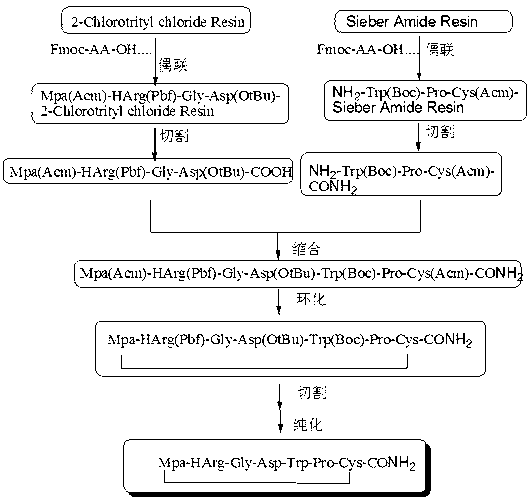
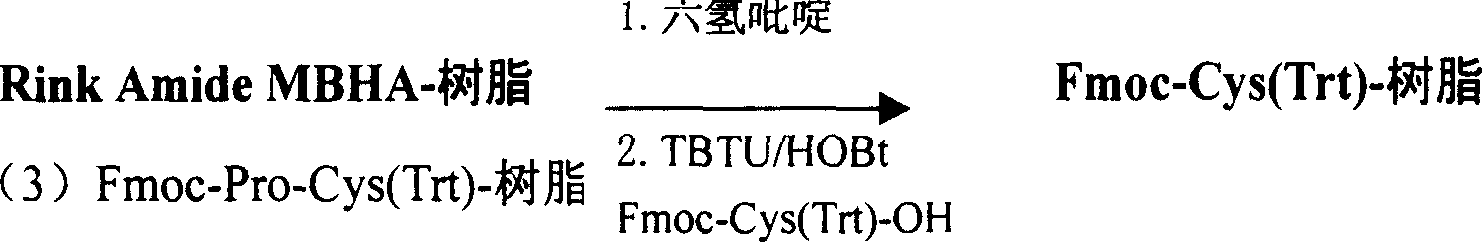
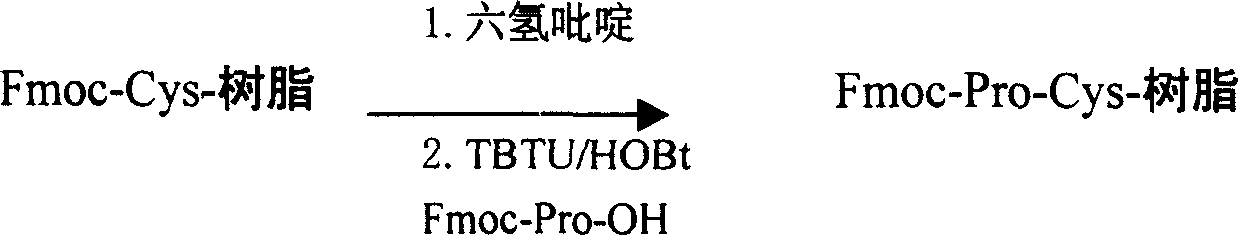
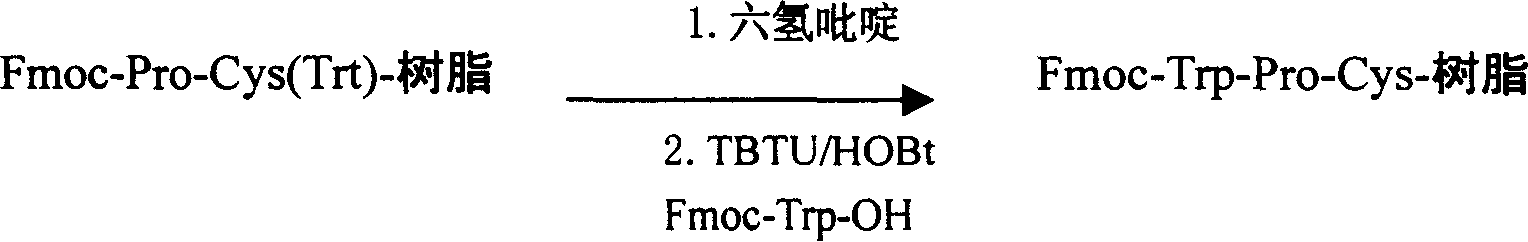
Preparing process for Eptifibatide
InactiveCN1500805AEasy to trackPromote safe productionPeptidesBlood disorderCyclic peptideTert-Butyloxycarbonyl protecting group
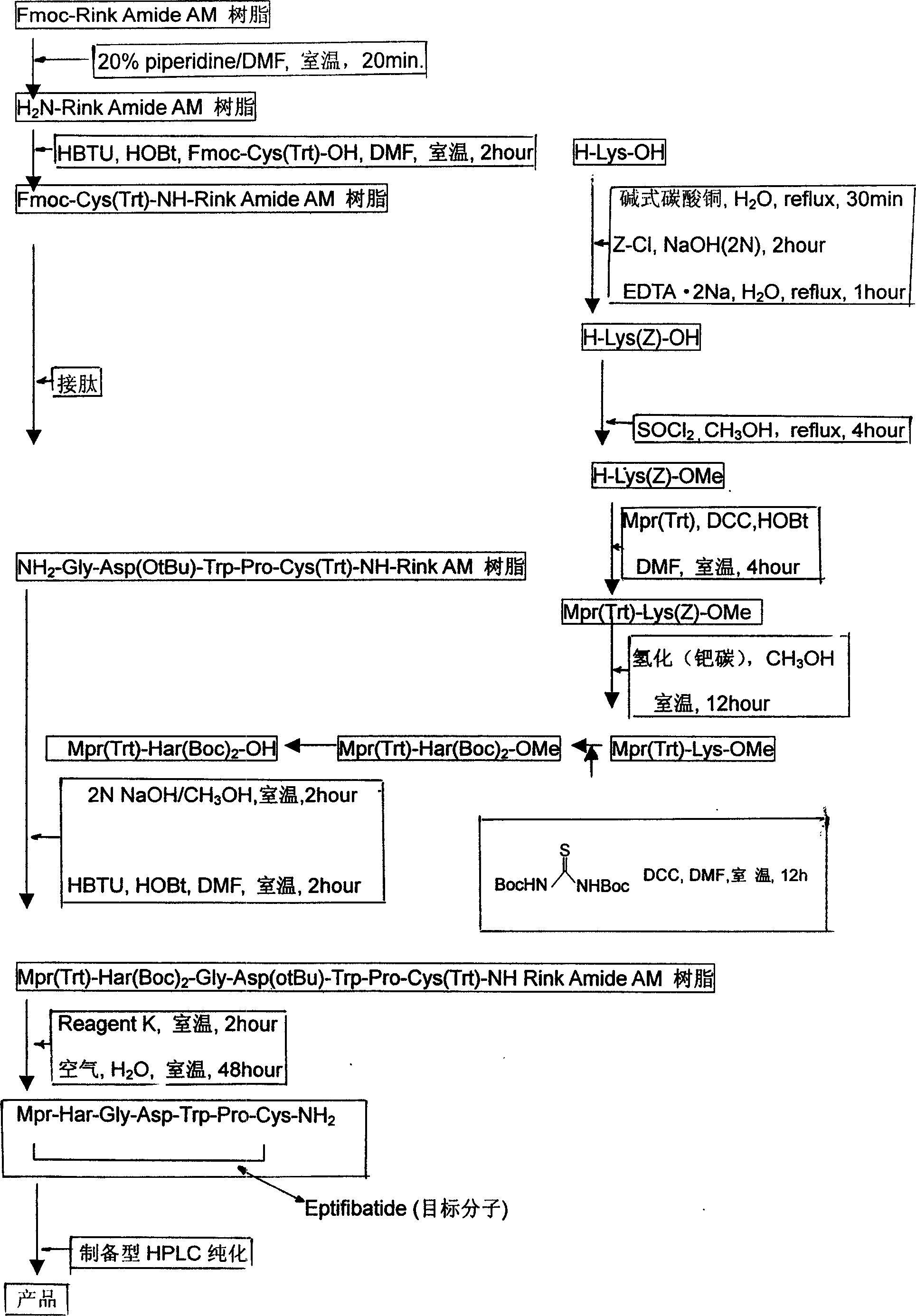
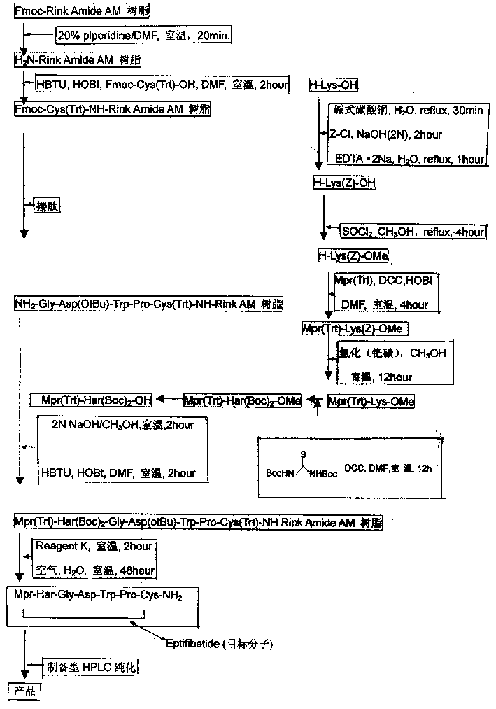
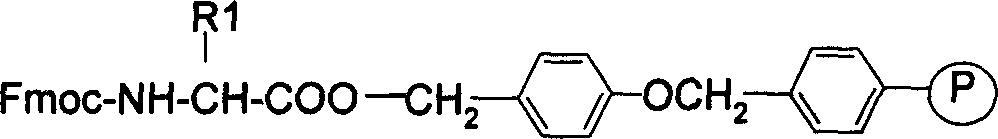
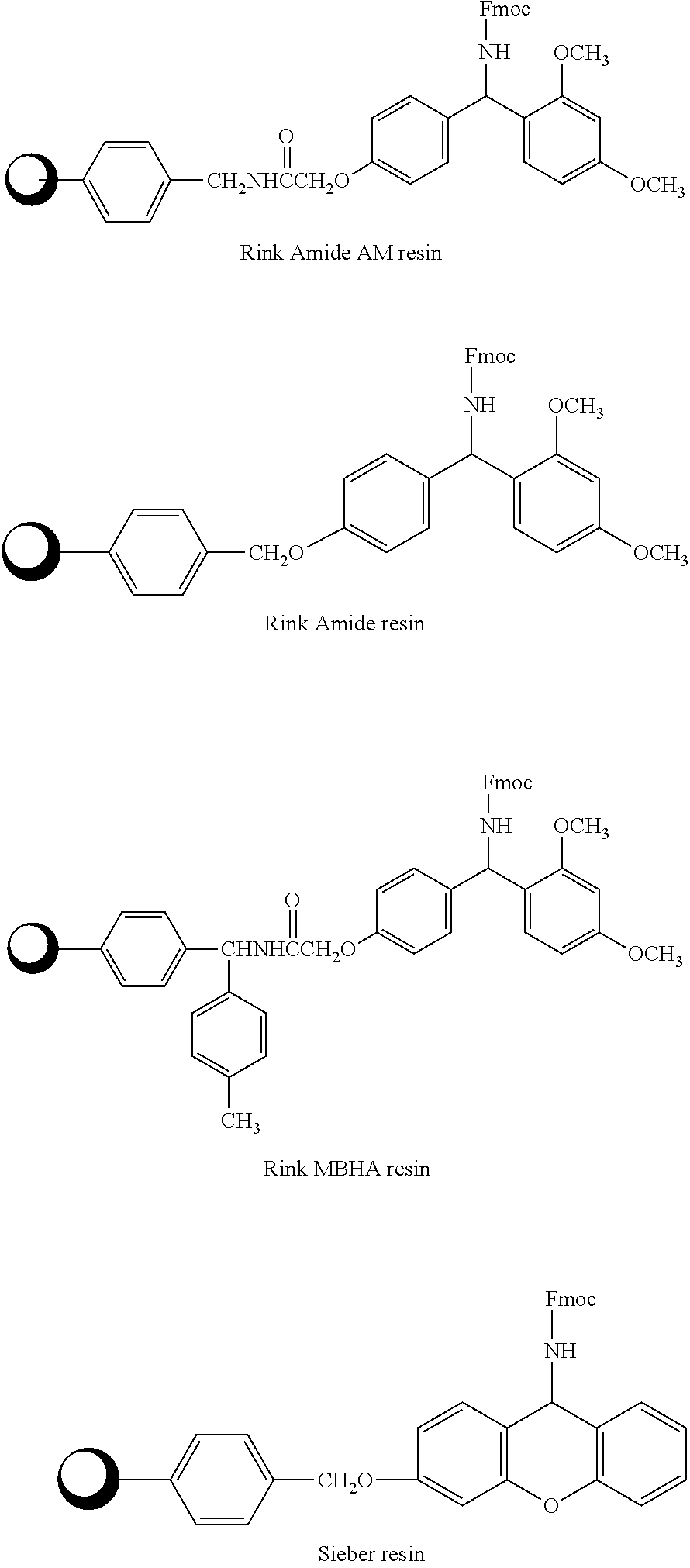
The present invention relates to the preparation of cyclic peptide, and is especially new Fmoc-solid phase process of preparing Eptifibatide. The new process is superior to liquid phase process, which has long synthesis period, and BOC solid phase process, which uses virulent and corrosive material. The technological scheme is that the Eptifibatide preparing process includes the following steps: eliminating Fmoc protection of Fmoc-Rink Amide AM resin to obtain H2N-Rink Amide AM resin; connecting various protective amino acids successively to obtain corresponding resin; eliminating Fmoc-protection radical and Kaiser test to detect reaction procedure; preparing S-triphenyl mercapto propionyl-N, N-ditert butyl oxycarbonyl-homoarginine with lysine; grafting S-triphenyl mercapto propionyl-N, N-ditert butyl oxycarbonyl-homoarginine; eliminating side chain protecting radical and resin to reduce into coarse product; and cyclization, oxidation, HPLC tracking purification to obtain pure product.
Owner:GL BIOCHEM SHANGHAI +1
Method for improving stability of polypeptide active pharmaceutical ingredients
ActiveCN106188218AChange particle shapeChange moistureOxytocins/vasopressinsThymosin peptidesOxytocinBivalirudin
The invention discloses a method for improving the stability of polypeptide active pharmaceutical ingredients. The polypeptide active pharmaceutical ingredients comprise but are not limited to bivalirudin, octreotide acetate, lanreotide acetate, eptifibatide or cetrorelix acetate, ganirelix acetate, degarelix, liraglutide, oxytocin, thymosin alpha1, leuprolide acetate, goserelin acetate, terlipressin or linaclotide. The method comprises the steps that after polypeptide drugs are salified, a polypeptide solution containing compensation ions is obtained, and a target polypeptide product is prepared through an ultralow temperature vacuum freeze-drying method. According to the method for improving the stability of the polypeptide active pharmaceutical ingredients, the problem that the polypeptide active pharmaceutical ingredients are prone to degradation after being placed for a long time is solved, the uniformity of the product is improved, and the drug risk is reduced.
Owner:SINOPEP ALLSINO BIOPHARMACEUTICAL CO LTD
Method for preparing Eptifibatide with solid phase method
InactiveCN101538316AMild reaction conditionsReduce pollutionPeptide preparation methodsBlood disorderSide chainCombinatorial chemistry
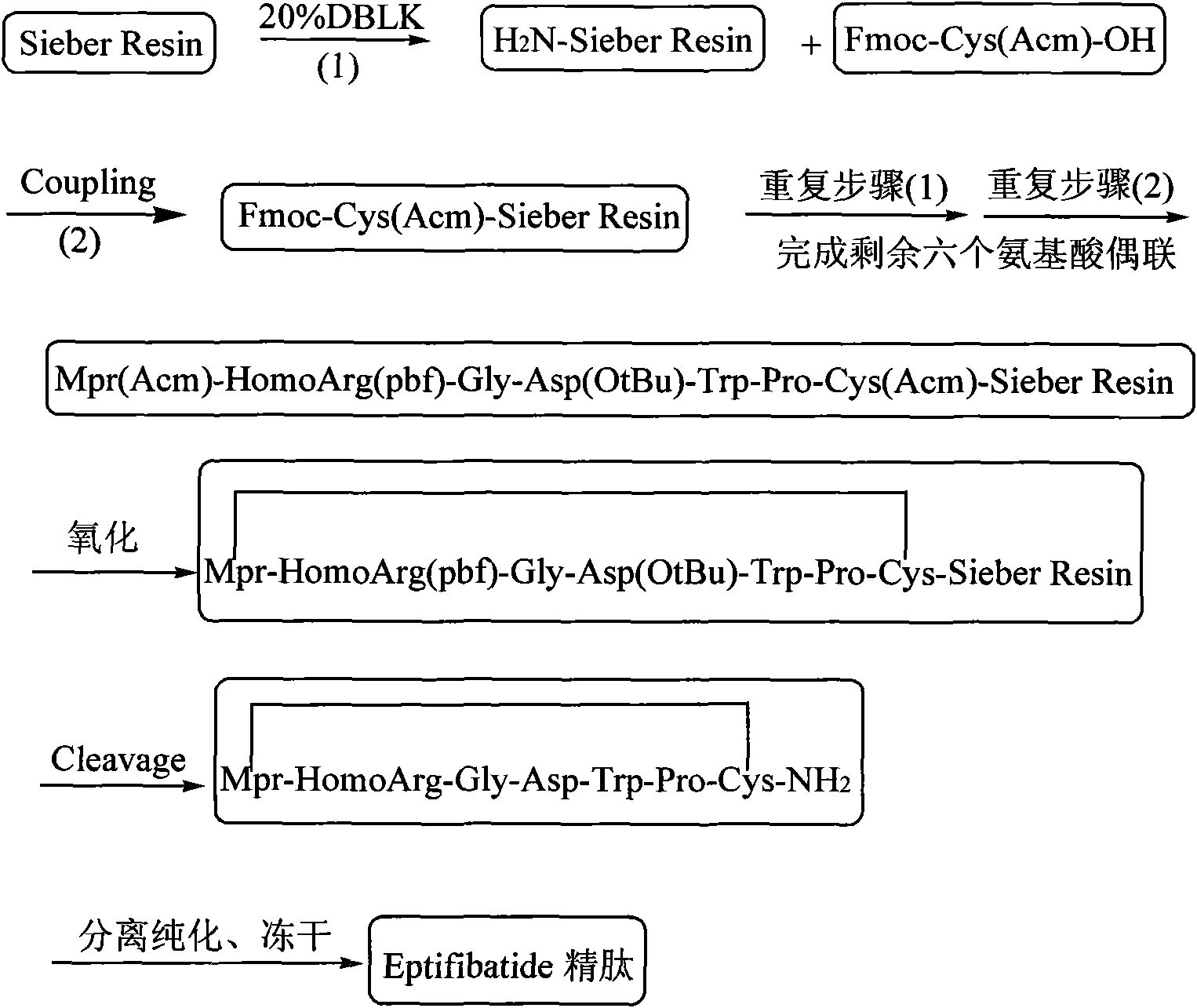
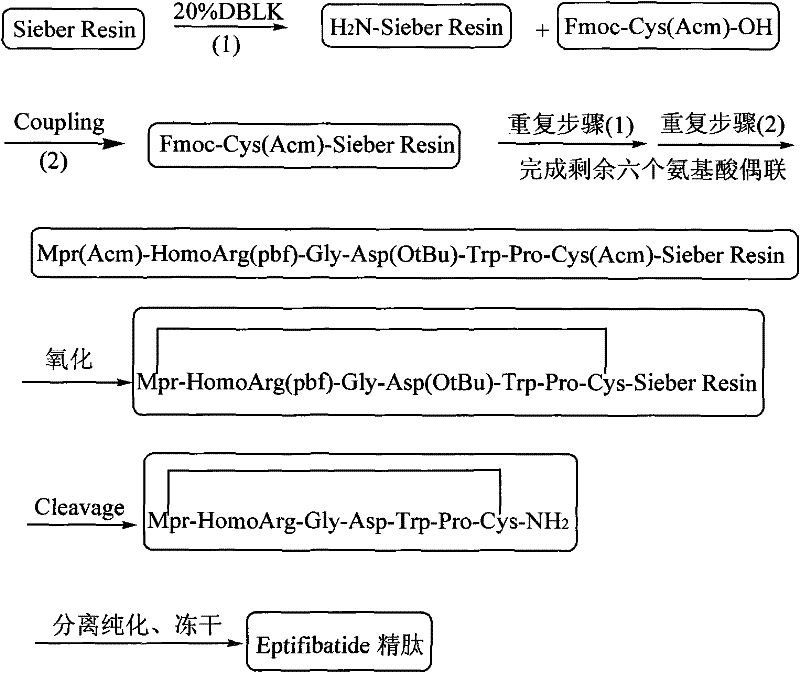
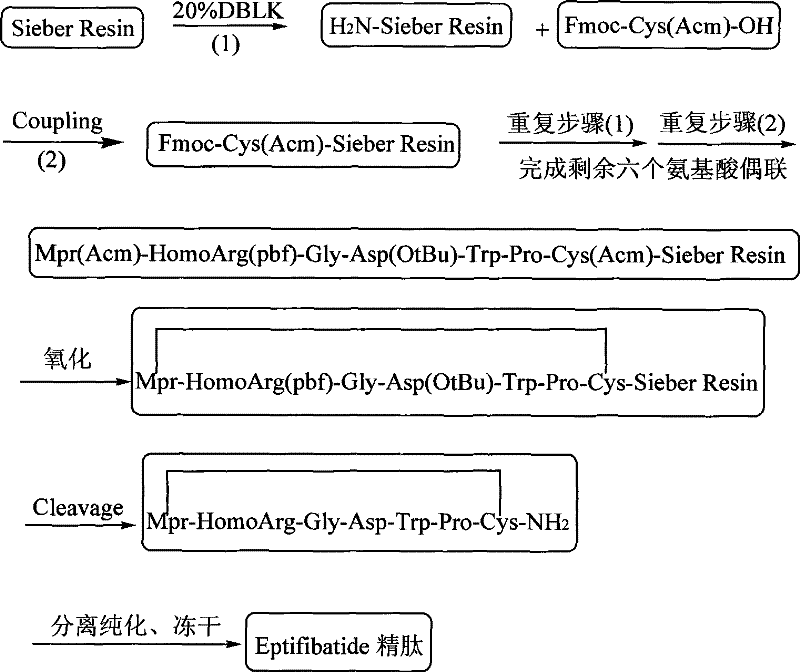
The invention discloses a method for preparing Eptifibatide with a solid phase method, which comprises the following steps of: 1) selecting Sieber resin to remove Fmoc, and obtaining H2N-Sieber resin; 2) adopting Fmoc / tBu solid phase method to couple and synthesize linear peptide Eptifibatide-Sieber resin with full protective lateral chains in sequence; 3) conducting solid phase oxidation to the resin, and obtaining oxidant peptide Eptifibatide-Sieber resin with full protective lateral chains; 4) cutting the resin and removing the lateral chain protection, and obtaining crude product of Eptifibatide; and 5) conducting separation and purification, and breeze drying by a freeze dryer, and obtaining refined Eptifibatide peptide. The technology is characterized by simple operation, easy post treatment, less investment of raw material, low cost, high yield and the like, and has considerable economical and practical value and wide application prospect in the field of polypeptide drug design and synthesis simultaneously.
Owner:HYBIO PHARMA
A method of purifying a peptide
InactiveCN101981048AHigh yieldCation exchanger materialsPeptide/protein ingredientsCyclic peptideCombinatorial chemistry
The invention relates, interalia, to the field of purification of peptides, notably cyclic or non-cyclic peptides their analogs or derivatives thereof. More particularly, the invention relates to a simplified and optimized purification process of cyclic peptides from a composition comprising the said peptide and at least one related impurity by chromatographic procedures enabling high yields, selectivity and purity of the desired end product. The improved process is particularly useful for the preparation of eptifibatide, exenatide, atosiban, nesiritide and their respective derivatives and analogs. The polypeptides are prepared in high purity of at least about 96 %, and preferably at least about 99 %.
Owner:BIOCON LTD
Method for preparing eptifibatide
InactiveCN102702320AReduces chances of exposure to alkaliReduce generationPeptide preparation methodsHplc methodCombinatorial chemistry
The invention relates to a method for preparing eptifibatide serving as a polypeptide medicament by combing solid and liquid phases, which belongs to the technical field of synthesis of polypeptides. According to the technical scheme of the invention, the method comprises the following steps of: (1) preparing a fragment A, i.e., 4peptide2-CTC resin by using a solid phase; (2) preparing a fragmentB, i.e., 3peptide-Sieber resin by using a solid phase; (3) cracking peptide resin; (4) preparing a fully-protected linear peptide from the fragments A and B by adopting a liquid phase fragment condensation method; (6) oxidizing with I2 in a liquid phase; (7) cracking to obtain crude eptifibatide; and (8) purifying the crude eptifibatide with a HPLC (High Performance Liquid Chromatography) method to obtain fine eptifibatide finally. According to the method, racemization products of Cys and Asp can be reduced greatly, and the yield and purity of a product are increased.
Owner:HYBIO PHARMA
Synthesis and preparation process of eptifibatide
InactiveCN101747412AHigh yieldLow costPeptide preparation methodsBulk chemical productionCyclic peptideCombinatorial chemistry
The invention provides synthesis and preparation process of eptifibatide, which includes the following steps: (1) liquid-phase synthesis of peptide segment 1: Mpr(x)-Har(R4)-Gly-OH; (2) liquid-phase synthesis of peptide segment 2: R1-Asp(R2)-Trp(R3)-Pro-Cys(Y)-NH2; (3) condensation of the peptide segment 1 and the peptide segment 2 to prepare fully protected peptide segment 3: Mpr(X)-Har(R4)-Gly-Asp(R2)-Trp(R3)-Pro-Cys(Y)-NH2; (4) deprotection of the peptide segment 3 to get linear peptide: Mpr(X)-Har-Gly-Asp-Trp-Pro-Cys(Y)-NH2; and (5) oxidation of the linear peptide to synthetize disulfide bridges to produce cyclic peptide. Through the method of firstly synthesizing linear peptide and then cyclizing the linear peptide, the invention substantially improves the yield, the product is obtained finally through purification, the cost is reduced, and the synthesis and preparation process is suitable for industrial production.
Owner:SINOPEP ALLSINO BIOPHARMACEUTICAL CO LTD
Solid-phase synthesis method for eptifibatide
ActiveCN101838308APromote oxidationShorten the production cyclePeptide preparation methodsBlood disorderAcetic acidFreeze-drying
The invention discloses a solid-phase synthesis method for eptifibatide. The solid-phase synthesis method for the eptifibatide comprises the following steps of: carrying out amino acid condensation on carrier resin, drying the obtained resin, and cutting the resin with trifluoroacetic acid to obtain an eptifibatide coarse product; oxidizing the eptifibatide coarse product with hydrogen peroxide for 30 to 60 minutes; neutralizing with acetic acid, filtering the mixture, carrying out HPLC purification on the filtrate, and collecting characteristic peaks; and distilling, concentrating and freeze-drying collections to obtain an acetic acid eptifibatide refined product. The invention provides a simple and easily operated process synthesizing route, and only needs monoprotected Fmoc-Har-OH2 and Fmoc-Trp-OH2 when raw materials are selected during synthesizing so as to easily obtain the raw materials and shorten production cycle.
Owner:YANCHENG KAILI PHARMA
Process for preparing solid phase polypeptide synthetic eptifibatide
ActiveCN1858060AConvenient sourceHigh peptide yieldPeptide preparation methodsPropanoic acidRink amide resin
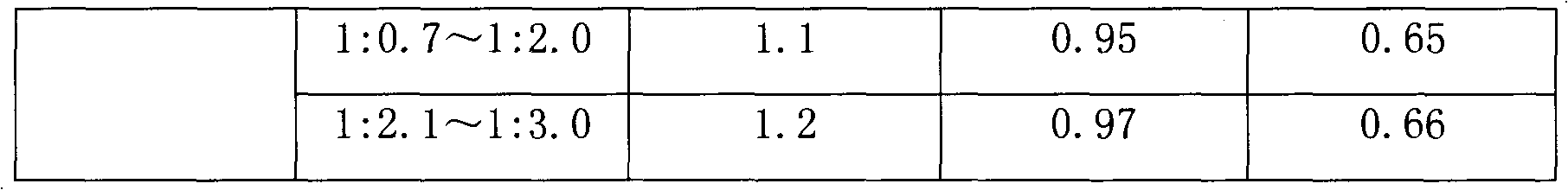
The preparation process of solid phase polypeptide synthesized eptifibatide includes the following steps: (1) connecting amino acids one by one with Rink Amide resin, Rink Amide MBHA resin or Rink Amide AM resin as initial material, and Fmoc protected amino aids as monomer, with the last peptide chain being S-benzyl mercapto propionic acid Map(SBzl); (2) adding peptide cutting agent (TFA / HBr / HAc / TIS / EDT) to cut peptide; (3) precipitating and collecting coarse reductant eptifibatide product in ether solvent; (4) dissolving coarse reductant eptifibatide product in water, regulating pH value with ammonia water to 7.5-10.0, introducing air for oxidation, and collecting coarse eptifibatide product; and (5) separating and purifying the coarse product in C18 column to obtain the target product. The present invention has high yield, and is suitable for industrial production.
Owner:SHANGHAI SOHO YIMING PHARMA
Method of Purifying a Peptide
InactiveUS20100317827A1High yieldPeptide/protein ingredientsSolid sorbent liquid separationCyclic peptidePurification methods
The invention relates, interalia, to the field of purification of peptides, notably cyclic or non-cyclic peptides their analogs or derivatives thereof. More particularly, the invention relates to a simplified and optimized purification process of cyclic peptides from a composition comprising the said peptide and at least one related impurity by chromatographic procedures enabling high yields, selectivity and purity of the desired end product. The improved process is particularly useful for the preparation of eptifibatide, exenatide, atosiban, nesiritide and their respective derivatives and analogs. The polypeptides are prepared in high purity of at least about 96%, and preferably at least about 99%.
Owner:BIOCON LTD
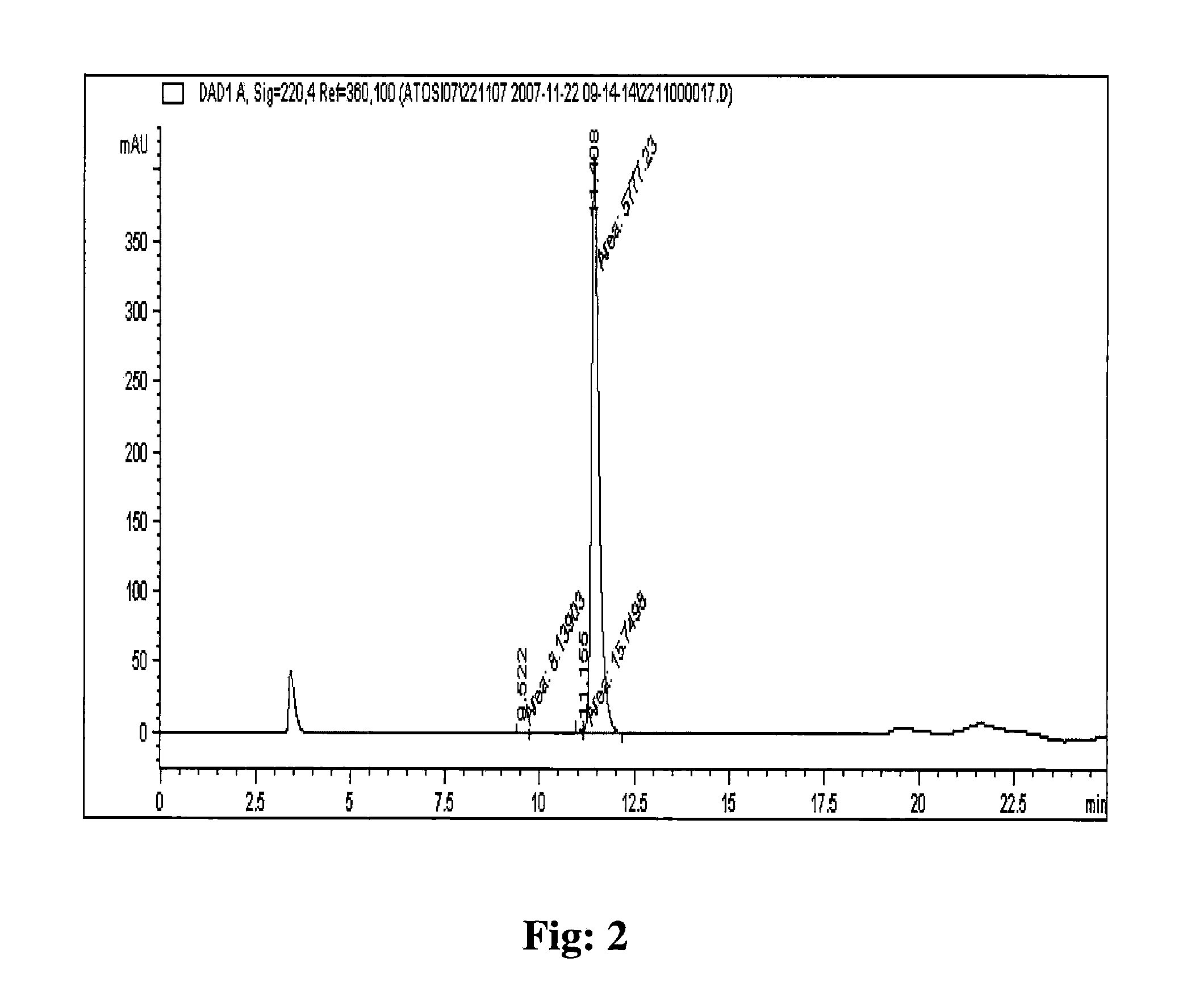
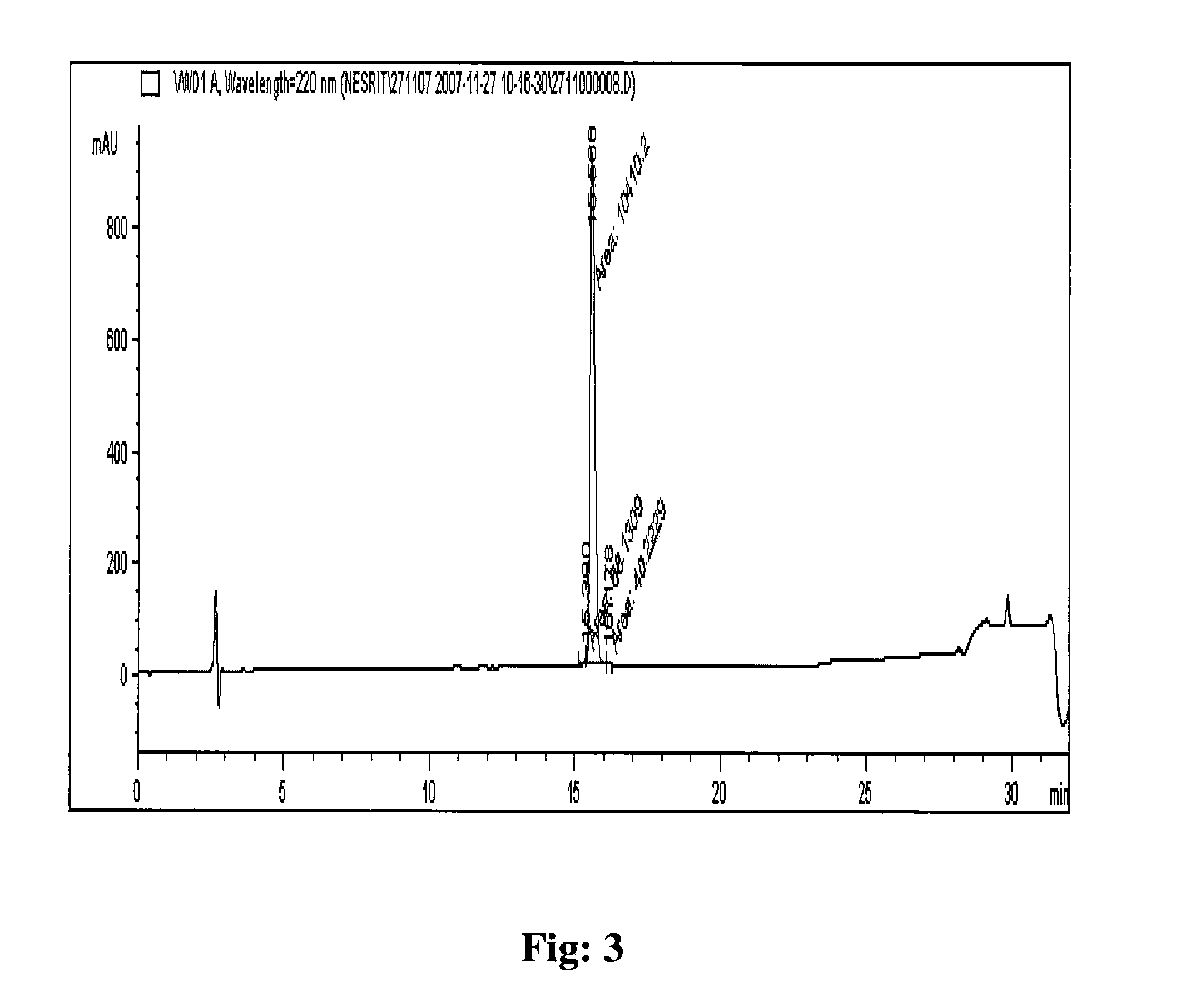
Processes for preparing eptifibatide
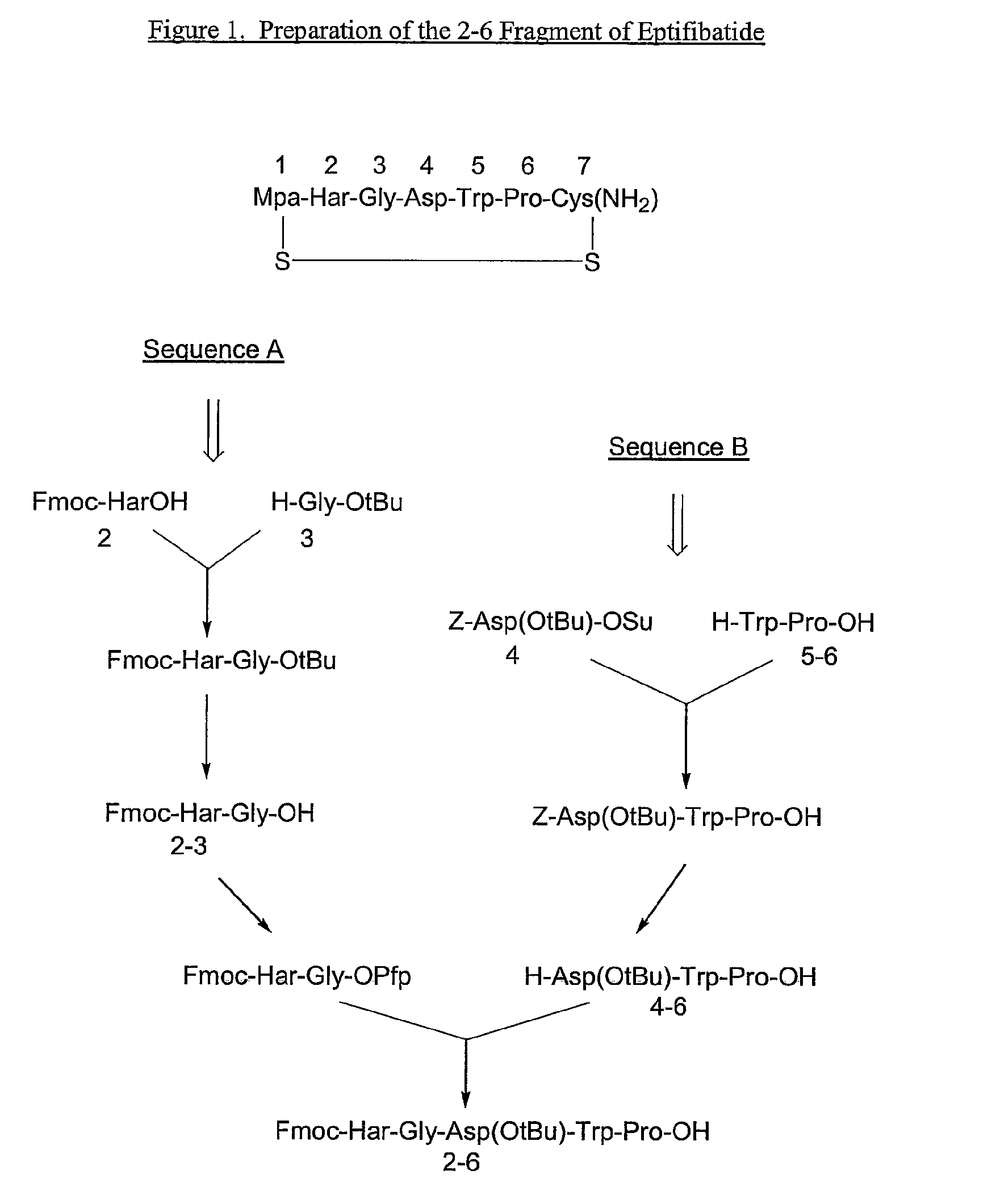
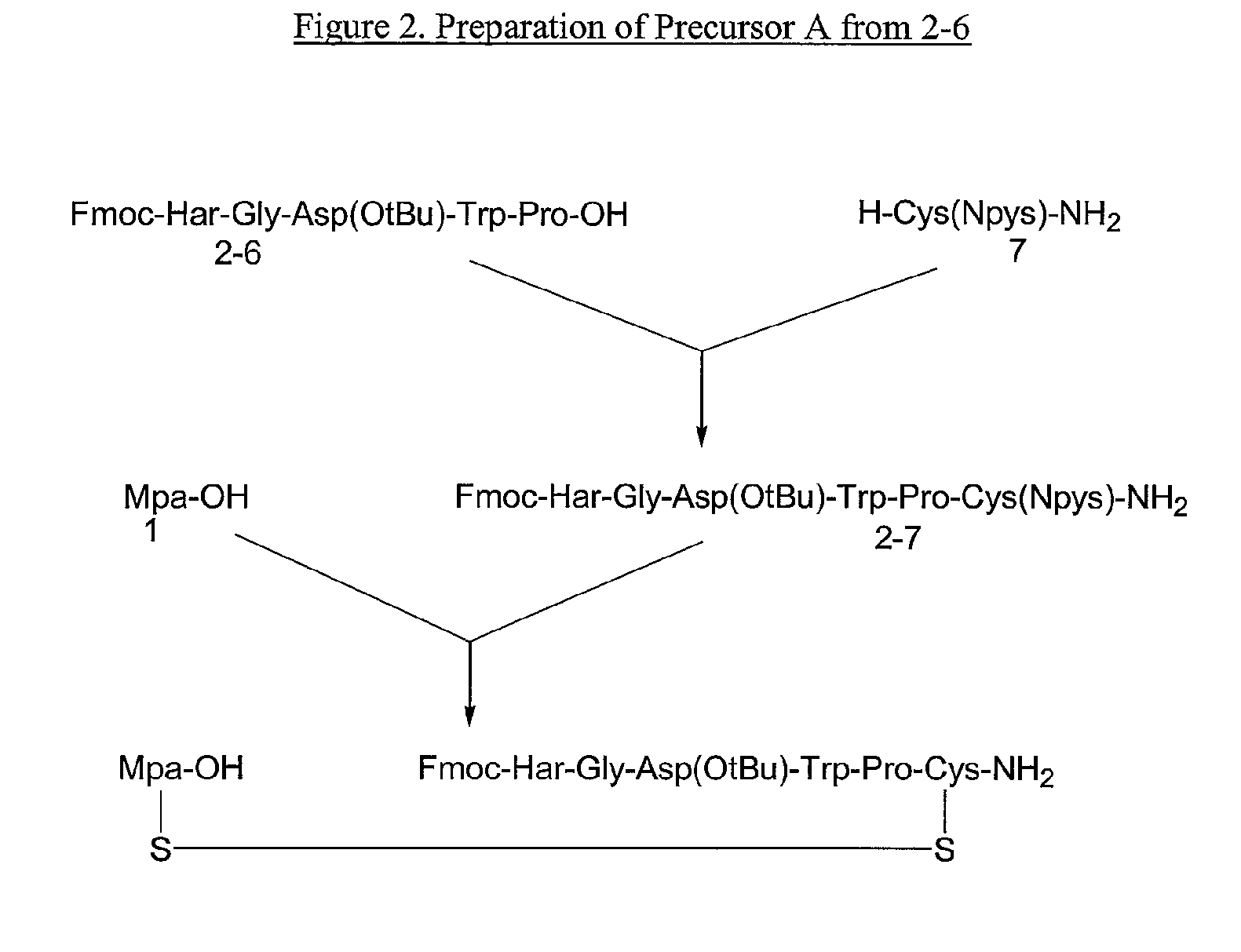
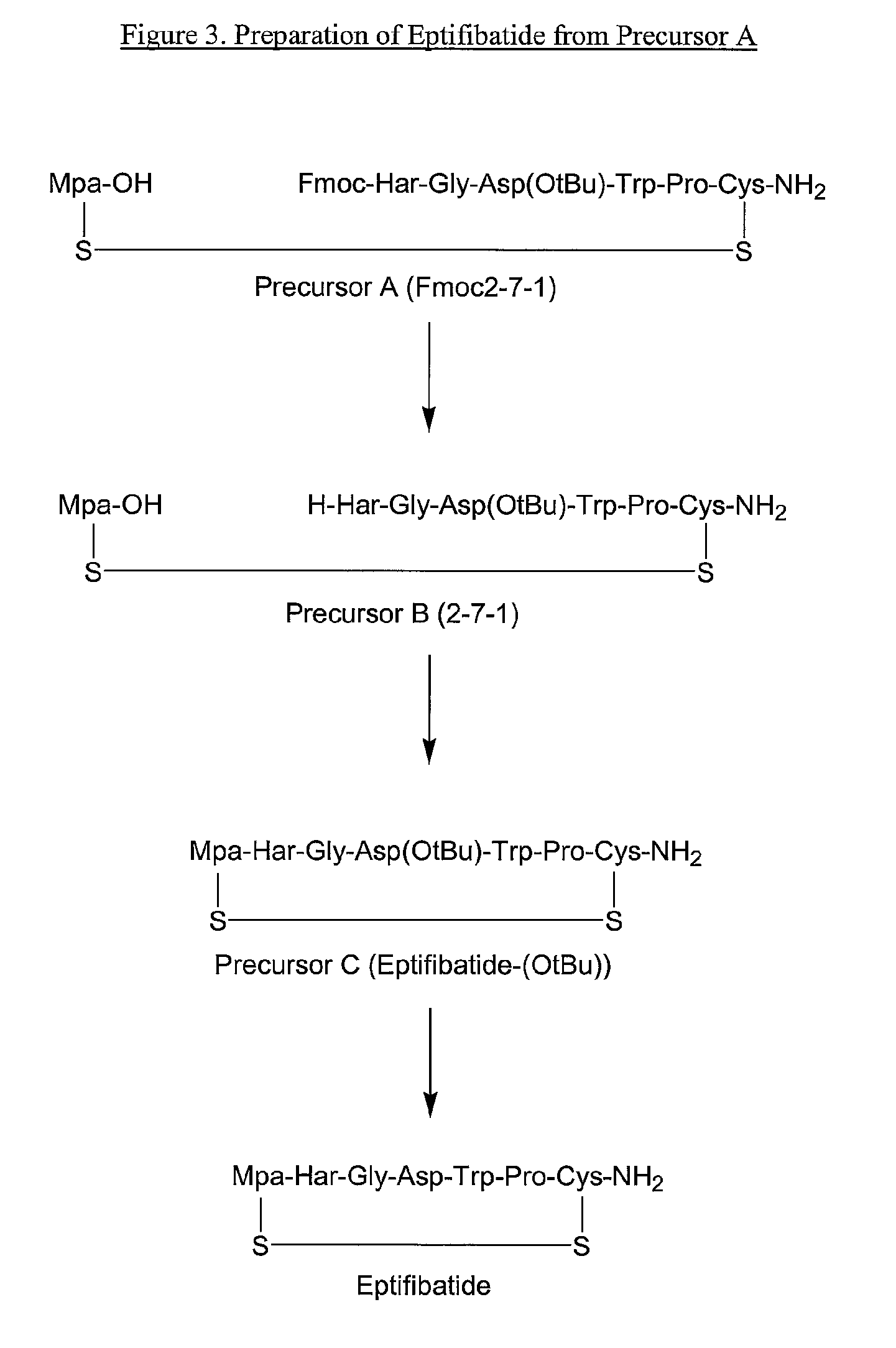
The present invention provides, inter alia, convergent processes for preparing eptifibatide that involve coupling a 2-6 eptifibatide fragment to an activated cysteinamide residue to form a 2-7 eptifibatide fragment, attaching a mercaptopropionic acid residue to the 2-7 eptifibatide fragment through disulfide bond formation, coupling the peptide intramolecularly, and removing the protecting group, to form eptifibatide. The invention further provides products produced by the described processes, novel compounds that can be used as synthetic intermediates for the preparation of eptifibatide, and novel compounds that are structurally similar to eptifibatide.
Owner:MILLENNIUM PHARMA INC
Method for synthesizing eptifibatide
InactiveCN101759776ASimple stepsEasy to operatePeptide preparation methodsBulk chemical productionDegree of substitutionCutting agent
The invention provides a method for synthesizing eptifibatide. The method comprises the steps of: (1) condensing Fmoc Rinklinker with AM resin having a degree of substitution of 0.8 to 1.2 mmol / g, so as to acquire a Fmoc-polypeptide resin, (2) mixing the Fmoc-polypeptide resin with a protective agent and removing the Fmoc protecting base so as to acquire a protected polypeptide resin, (3) condensing the protected amino acid of the Fmoc with the protected polypeptide resin by using the condensing agent, (4) repeating the step (2) to step (3) so as to acquire a hexapeptide resin, (5) condensing the hexapeptide resin with Mpa(Trt)-OH so as to acquire a heptamer resin, (6) separating the heptamer from the resin by using cutting agent so as to acquire the crude product of eptifibatide, and (7) oxidizing and cyclizing the crude product of eptifibatide in order to acquire the eptifibatide. The method of the invention can promote the yield of the eptifibatide, decrease the cost and promote the purity.
Owner:SHANGHAI AMBIOPHARM
Method for purifying Eptifibatide
ActiveCN101538314AHigh purityHigh yield purification yieldPeptide preparation methodsBlood disorderAcetic acidBüchner funnel
The invention discloses a method for purifying Eptifibatide, which comprises the following steps of: 1) conducting suction filtration with a Buchner funnel containing diatomite to remove slightly soluble impurities; 2) conducting gradient elution and purification with the fixed phase being alkylsilane bonded silica, the phase A of a mobile phase being trifluoroacetic acid aqueous solution and the phase B thereof being chromatographic grade acetonitrile, wherein the mobile phase includes phase A and phase B; and collecting the peptide solution at a target peak value, and conducting reduced pressure distillation and concentration; and 3) using anion exchange method for converting the trifluoroacetate into acetate, thus obtaining the acetic acid Eptifibatide bulk drug which meets the requirement. In the invention, the method which is applicable to the industrialized purification of Eptifibatide is provided; reversed phase high-performance liquid chromatography is used and combined with the anion exchange method for converting the trifluoroacetate into acetate so as to purify the Eptifibatide; and the purity is high, and the purification yield of the yield reaches more than 70 percent and meets the industrialization requirement.
Owner:HYBIO PHARMA
Preparation method of eptifibatide
The invention relates to the field of preparation of polypeptides and particularly relates to a preparation method of eptifibatide. The preparation method comprises the following steps of: preparing a fine peptide solution of the eptifibatide; and performing rotary evaporation on the fine peptide solution of the eptifibatide to get a fine peptide concentrated solution of the eptifibatide, and freeze-drying to obtain eptifibatide, wherein the concentration of the fine peptide concentrated solution of the eptifibatide is 15-30mg / mL; and the temperature of rotary evaporation is 25 DEG C-35 DEG C. The preparation method of the fine peptide solution of the eptifibatide comprises the following steps of: coupling Cys with resin to get Cys-resin; and gradually coupling the Cys-resin with Pro, Trp, Asp, Gly, Har and Mpr to get a peptide with the sequence as shown in SEQ (sequence) ID (identity) NO: 1, and further performing oxidation, cracking, purification and salt transfer to get the fine peptide solution of the eptifibatide. By adopting the preparation method provided by the invention, the production of dimer impurities in the eptifibatide can be greatly reduced, and organic residues of acetonitrile and the like can be in line with the related standard in Chinese Pharmacopoeia.
Owner:HYBIO PHARMA
Eptifibatide preparation method
ActiveCN104710509AReduce generationReduce solubilityPeptide preparation methodsBulk chemical productionSolubilityRink amide resin
The invention relates to the field of polypeptide medicine preparation, and especially relates to an eptifibatide preparation method. The method comprises the following steps: synthesizing Fmoc-Cys(x)-Rink amide Resin; sequentially coupling -Pro, -Trp, -Asp, -Gly, -Har and -Mpr to the Fmoc-Cys(x)-Rink amide Resin through adopting a solid phase synthesis technology according to the peptide sequence of eptifibatide to prepare eptifibatide linear resin, and cracking to prepare linear eptifibatide; and taking the linear eptifibatide, oxidizing to prepare crude eptifibatide, and purifying to obtain eptifibatide. A low temperature reaction makes the single impurity content below 0.15%. Liquid phase oxidation is carried out to adjust the pH value to 7.2-7.5, so the oxidation rate is guaranteed, and the solubility of the eptifibatide product in an oxidation liquid is reduced, thereby a problem of difficult recovery due to massive dissolution is avoided. The purity of eptifibatide prepared through the method is greater than 99%, and the single impurity content is smaller than 0.15%.
Owner:HYBIO PHARMA
Liquid-phase synthesis method of eptifibatide
ActiveCN103450346AImprove cyclization efficiencyLess side effectsPeptide preparation methodsBulk chemical productionSide chainSynthesis methods
Owner:山东博创生物科技有限公司
Eptifibatide preparing method
InactiveCN105218641AMild acid hydrolysis conditionsAvoid it happening againPeptide preparation methodsDrugs synthesisImpurity
The invention belongs to the field of polypeptide drug synthesis, and particularly relates to an eptifibatide preparing method. The method comprises the steps of 1, conducting coupling sequentially by means of the polypeptide solid-phase synthesis method with protected amino acid, fragments and amino resin as raw materials to obtain eptifibatide peptide resin X-Gly-Asp(OtBu)-Trp(Boc)-pro-Cys(R1)-amino resin, wherein R1 is Trt, Mtt, Mmt or Dmt, X is Mpr(R2)-Harg, and R2 is Mtt, Mmt or Dmt; 2, adding an acidolysis reagent to the eptifibatide peptide resin for acidolysis to obtain a reduced eptifibatide crude product; 3, oxidizing the reduced eptifibatide crude product to obtain an eptifibatide crude product; 4, conducting separation and purification on the eptifibatide crude product through a C18 column to obtain eptifibatide. The method has the advantages that due to the adoption of Fmoc-Cys(R1) and the fragment Mpr(R2)-Harg, usage of the expensive raw material Fmoc-Harg(Pbf)-OH is avoided, peptide resin acidolysis condition is made milder, generation of impurities can be effectively avoided, product purity can be over 99.5%, and industrial prospects are broad.
Owner:叶仲林
Method for preparing Eptifibatide with solid phase method
InactiveCN101538316BMild reaction conditionsReduce pollutionPeptide preparation methodsBlood disorderSide chainCombinatorial chemistry
The invention discloses a method for preparing Eptifibatide with a solid phase method, which comprises the following steps of: 1) selecting Sieber resin to remove Fmoc, and obtaining H2N-Sieber resin; 2) adopting Fmoc / tBu solid phase method to couple and synthesize linear peptide Eptifibatide-Sieber resin with full protective lateral chains in sequence; 3) conducting solid phase oxidation to the resin, and obtaining oxidant peptide Eptifibatide-Sieber resin with full protective lateral chains; 4) cutting the resin and removing the lateral chain protection, and obtaining crude product of Eptifibatide; and 5) conducting separation and purification, and breeze drying by a freeze dryer, and obtaining refined Eptifibatide peptide. The technology is characterized by simple operation, easy post treatment, less investment of raw material, low cost, high yield and the like, and has considerable economical and practical value and wide application prospect in the field of polypeptide drug design and synthesis simultaneously.
Owner:HYBIO PHARMA
Preparation method for eptifibatide
ActiveCN105037496AAvoid it happening againReduce the difficulty of purificationPeptide preparation methodsFreeze-dryingAcetonitrile
The invention discloses a preparation method for eptifibatide. The method includes the steps of sequentially coupling and connecting Fmoc-protected amino acid and a protected amino acid tripeptide section with Fmoc-Cys(Trt) resin as a coupling resin carrier, splitting eptifibatide resin through a split agent, oxidizing reduced type eptifibatide in an acetonitrile aqueous solution, conducting condensation after oxidation, and conducting purification, salt transfer and freeze drying on crude eptifibatide product concentrated liquor to obtain a fine eptifibatide product. By means of the preparation method, production cost can be effectively reduced, yield is increased, and the content of impurities in the finished product is reduced.
Owner:SICHUAN JISHENG BIOPHARM CO LTD
Novel process for preparing eptifibatide by purification
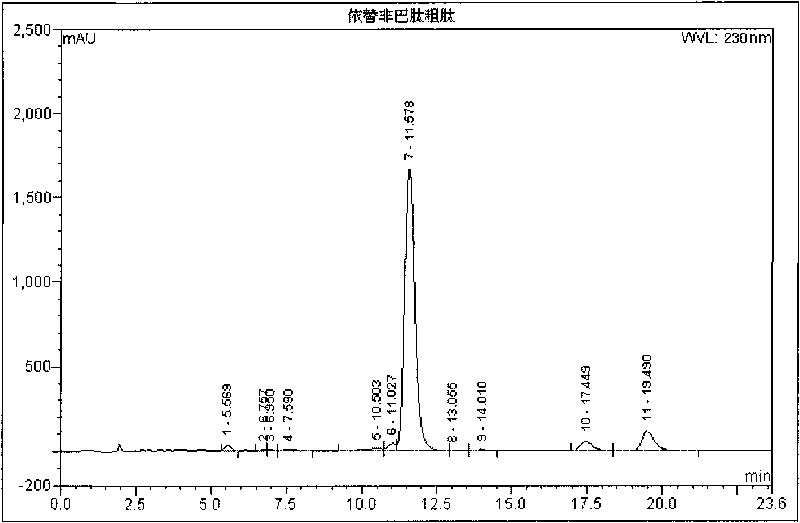
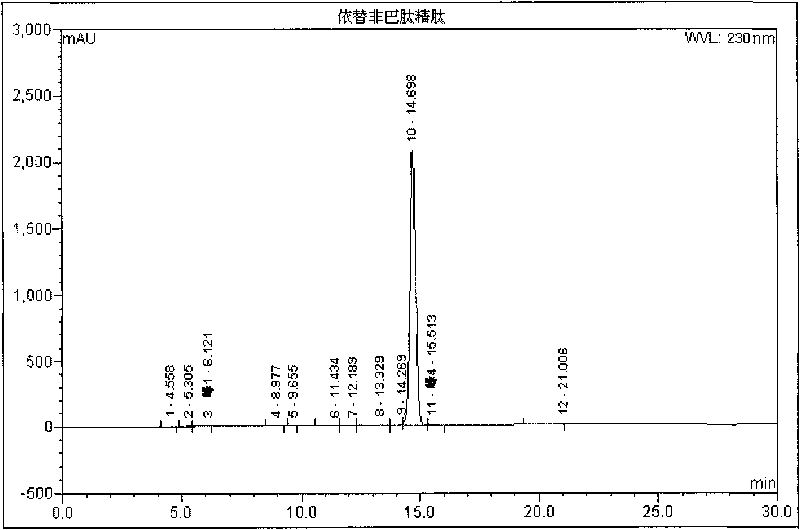
InactiveCN101372506AReduce processing difficultyEase of industrial scale productionPeptide preparation methodsUv detectorIon exchange
The invention relates to a new technique for purifying and preparing eptifibatide. At present, the eptifibatide is separated, purified and produced by adopting opposite phase high pressure liquid chromatography. However, mass production is not easy to realize, and the equipment are expensive. Separation and purification are carried out by the method by applying two solvent systems which are not dissolved in each other and make epicyclic motion at high speed in a chromatographic column tube; the processing steps comprises: a. synthetic crude product of the eptifibatide is dissolved by solvent; b. the dissolved crude product of the eptifibatide is separated and purified by high-speed centrifugation separation chromatography (FCPC), and is tested by a uv detector from the distance of 230nm, so that target peak is collected by subsection; c. the collected cut fraction is tested by HPLC, wherein, the cut fraction with the purity higher than 98% is bended to be treated by the next step, and the cut fraction with the purity lower than 98% is recycled and purified again; d. ion exchange is carried out on the cut fraction with qualified purity to remove trifluoroacetic acid (TFA), and then the cut fraction is transformed into acetic acid eptifibatide. The new technique has no irreversible adsorption and the advantages of no loss of sample, no pollution, high speed and high efficiency, and is suitable for mass production.
Owner:HYBIO PHARMA
Method for preparing eptifibatide and precursor thereof
ActiveCN102174081AReduce usageHigh yieldPeptide preparation methodsBulk chemical productionCyclic peptideSynthesis methods
The invention discloses a method for synthesizing eptifibatide, which comprises the following steps of: synthesizing the 1-3 fragment and 4-7 fragment of the eptifibatide, coupling the two fragments by using a disulfide bond to obtain the 4-7-1-3 fragment of the eptifibatide, intramolecularly combining to form a cyclic peptide, and removing a protection group to form the eptifibatide. A liquid phase synthesis method is adopted, and a ring is formed through a molecular lactam bond, so the yield of the eptifibatide is higher than that of the eptifibatide through the ring formation of the disulfide bond, total cost is obviously reduced, and expensive resin is avoided.
Owner:HANGZHOU HUADI GRP CO LTD
Eptifibatide preparation method
An Eptifibatide preparation method with product purity of more than 99.5%, the method comprising: using a solid phase polypeptide synthesis method to prepare eptifibatide resin, conducting acidolysis on the Eptifibatide resin to obtain a crude Eptifibatide linear peptide product, oxidizing to obtain a crude Eptifibatide product, purifying and exchanging salt to obtain an Eptifibatide finished product; the method using the solid phase polypeptide synthesis method to prepare the eptifibatide resin is: using a solid phase coupling synthesis method to sequentially splice a corresponding protective amino acid or a segment in the following sequence onto amino resin, and obtaining the Eptifibatide resin: X—Y-Trp(R1)-Pro-Cys(R2)-amino resin, wherein R1 is Boc or H, R2 is Trt or Acm, X is Mpr(R2)-Harg(R3), R3 is Pbf or H, and Y is Gly-Asp(OtBu).
Owner:CHENGDU SHENGNUO BIOTEC CO LTD
Eptifibatide synthesis method
InactiveCN105585613AShorten the timeShorten the production cyclePeptide preparation methodsGlycinePropanoic acid
The invention discloses an eptifibatide synthesis method. Fluorenylmethoxycarbonyl aminomethyl resin is used as a carrier, the method comprises amino acid condensation reactions from Step 1 to Step 7, resin connected with 7 amino acids is cut and linear thiohydracrylic acid-homoarginine-glycine-aspartic acid-tryptophan-proline-cysteine-NH2 is obtained in Step 8, a ring is formed under the catalysis of 2,2'-dipyridyl disulfide in Step 9, and eptifibatide is obtained. Further, the condensation reactions and deprotection reactions are conducted at 60-80 DEG C. The method has the characteristics of high yield, few byproduct and simplicity in separation and purification, and compared with the prior art, the method has the advantages that a lot of time is saved and the method is applicable to pilot production and industrial production.
Owner:何润泽
Eptifibatide lyophilized powder injection with superior rehydration
InactiveCN102204890AFast dissolutionImprove dissolution ratePowder deliveryCyclic peptide ingredientsDrugs solutionHigh doses
The invention belongs to the field of pharmaceutical preparations, and relates to an eptifibatide lyophilized powder injection formula and preparation thereof. The eptifibatide lyophilized powder injection is composed of eptifibatide and pharmaceutically acceptable auxiliary materials, contains a given amount of citric acid, excipient, acidity regulator and water for injection, and is prepared by preparing the drug and the auxiliary materials into a solution by the water for injection, and performing a lyophilizing process (the water for injection is removed in the process). The eptifibatide lyophilized powder injection provided by the invention solves the problems of high viscosity, long lyophilizing process and poor rehydration of a high dose of polypeptide drug solution, and provides convenience for clinical medication.
Owner:杨毅跃
Long-acting liposome preparation for pulmonary drug delivery and preparation method thereof
ActiveCN103784403AAntibody ingredientsCyclic peptide ingredientsPolyethylene glycolBULK ACTIVE INGREDIENT
The invention belongs to the field of pharmaceutical preparations, and relates to a long-acting liposome preparation for pulmonary drug delivery and a preparation method thereof. Specifically, the preparation takes polyethylene glycol modified liposome as a drug carrier, takes polypeptide drugs for treating cardiovascular diseases as active ingredients, and mainly comprises related peptides such as bivalirudin, eptifibatide, carperitide, nesiritide, romiplostim, and the like. The long-acting liposome preparation prepared according to the invention is high in encapsulation efficiency, easy to use, long in acting time and high in bioavailability.
Owner:SHENZHEN JYMED TECH
Process for preparing solid phase polypeptide synthetic eptifibatide
ActiveCN1858060BConvenient sourceHigh peptide yieldPeptide preparation methodsPropanoic acidRink amide resin
The preparation process of solid phase polypeptide synthesized eptifibatide includes the following steps: (1) connecting amino acids one by one with Rink Amide resin, Rink Amide MBHA resin or Rink Amide AM resin as initial material, and Fmoc protected amino aids as monomer, with the last peptide chain being S-benzyl mercapto propionic acid Map(SBzl); (2) adding peptide cutting agent (TFA / HBr / HAc / TIS / EDT) to cut peptide; (3) precipitating and collecting coarse reductant eptifibatide product in ether solvent; (4) dissolving coarse reductant eptifibatide product in water, regulating pH value with ammonia water to 7.5-10.0, introducing air for oxidation, and collecting coarse eptifibatide product; and (5) separating and purifying the coarse product in C18 column to obtain the target product. The present invention has high yield, and is suitable for industrial production.
Owner:SHANGHAI SOHO YIMING PHARMA
Composition for treating obesity and preparation method thereof
InactiveCN109893643AWeight lossObvious functionHydroxy compound active ingredientsPeptide/protein ingredientsMedicineBlood lipids
The invention discloses composition for treating obesity and a preparation method thereof. The composition comprises following components: radix angelicae sinensis, rhubarb, rhizoma alismatis, foliumnelumbinis, glycerine, plant essential oil and eptifibatide. The preparation method of the composition comprises following steps: mixing the radix angelicae sinensis, the rhubarb and the rhizoma alismatis uniformly, adding water, after boiling, performing filtrationto obtain boiled water, performing cooling, after adding the eptifibatide, the glycerine and the plant essential oil, mixing and stirring the mixture uniformly, performing homogenization, immersing a non-woven cloth sheet, and performing drying at low temperature after immersing to obtain the composition. The composition has a significant weight-loss effect and an auxiliary function of lowering blood lipid, and the effect is significant.
Owner:南京星银药业集团有限公司
Preparation method for eptifibatide anticoagulant drug
InactiveCN109305995AMild reaction conditionsHigh yieldPeptide preparation methodsBlood disorderAnticoagulant drugTetrapeptide
The invention discloses a preparation method for an eptifibatide anticoagulant drug and belongs to the technical field of synthesis of drug intermediates. The invention is characterized in that by utilizing a polypeptide condensing agent A, step 1, a tripeptide fragment Mpa (Trt)-Lys (Boc)-Gly-OH is synthesized firstly; step 2, a tetrapeptide fragment H-Asp (OTBS)-Trp-Pro-Cys (Trt)-NH2 is synthesized; and step 3, a disulfide bond is formed firstly to form a linear peptide, then condensation is performed to form cyclopeptide, and a guanidine group is formed finally to prepare eptifibatide as atarget product. The technology is high in yield as well as simple and convenient to treat; and the prepared eptifibatide is high in purity of raw materials and is suitable for industrial production.
Owner:重庆科脉生物化工有限公司
Method for preparing eptifibatide
Provided is a method for preparing eptifibatide, wherein the method comprises: obtaining an eptifibatide refined peptide solution; and obtaining and freeze-drying an eptifibatide refined peptide concentrate after rotary evaporation of the eptifibatide refined peptide solution, wherein the concentration of the eptifibatide refined peptide concentrate is 15-30 mg / ml and the temperature of rotary evaporation is 251° C.-35° C. The preparation method of the eptifibatide refined peptide solution is as follows: coupling Cys with a resin to obtain a Cys-resin; obtaining a polypeptide having a sequence as represented by SEQ ID NO: 1 by gradually coupling the Cys-resin with Pro, Trp, Asp, Gly, Har and Mpa; and obtaining the eptifibatide refined peptide solution through oxidation, cleavage, purification and transfer to salt.
Owner:HYBIO PHARMA
Platelet aggregation inhibition preparation and preparation method thereof
InactiveCN101780267ARealize regulationAchieve antioxidant propertiesPeptide/protein ingredientsPharmaceutical non-active ingredientsCurative effectBULK ACTIVE INGREDIENT
The invention discloses a platelet aggregation inhibition preparation, which belongs to the technical field of biomedicine. The platelet aggregation inhibition preparation comprises active ingredients and an auxiliary material, wherein the concentration of the active ingredients is 0.1-50 mg / ml, and the active ingredients are eptifibatide and pharmaceutically-acceptable eptifibatide derivatives; the auxiliary material is a buffer system of citric acid and sodium citrate; and in the buffer system, the concentrations of the citric acid and the sodium citrate are respectively 0.5-50 mg / ml and 0.5-50 mg / ml. The six-month accelerated test and the 24-month long-term stability test prove that the active ingredients are stable in the injection prepared from the eptifibatide preparation. In particular, the small volume injection prepared from the eptifibatide preparation has the advantages of good stability and accurate curative effect, and can maintain good stability during storage and transportation.
Owner:HYBIO PHARMA
Processes for preparing eptifibatide
The present invention provides, inter alia, convergent processes for preparing eptifibatide that involve coupling a 2-6 eptifibatide fragment to an activated cysteinamide residue to form a 2-7 eptifibatide fragment, attaching a mercaptopropionic acid residue to the 2-7 eptifibatide fragment through disulfide bond formation, coupling the peptide intramolecularly, and removing the protecting group, to form eptifibatide. The invention further provides products produced by the described processes, novel compounds that can be used as synthetic intermediates for the preparation of eptifibatide, and novel compounds that are structurally similar to eptifibatide.
Owner:MILLENNIUM PHARMA INC
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com