Preparing process for Eptifibatide
A new process and resin technology, applied in the direction of blood diseases, extracellular fluid diseases, peptides, etc., can solve the problems affecting the purity of crude products, the reaction is not easy to complete, and the difficulty of purification is increased, so as to achieve easy tracking, production cost reduction, and synthesis process The effect of mild conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
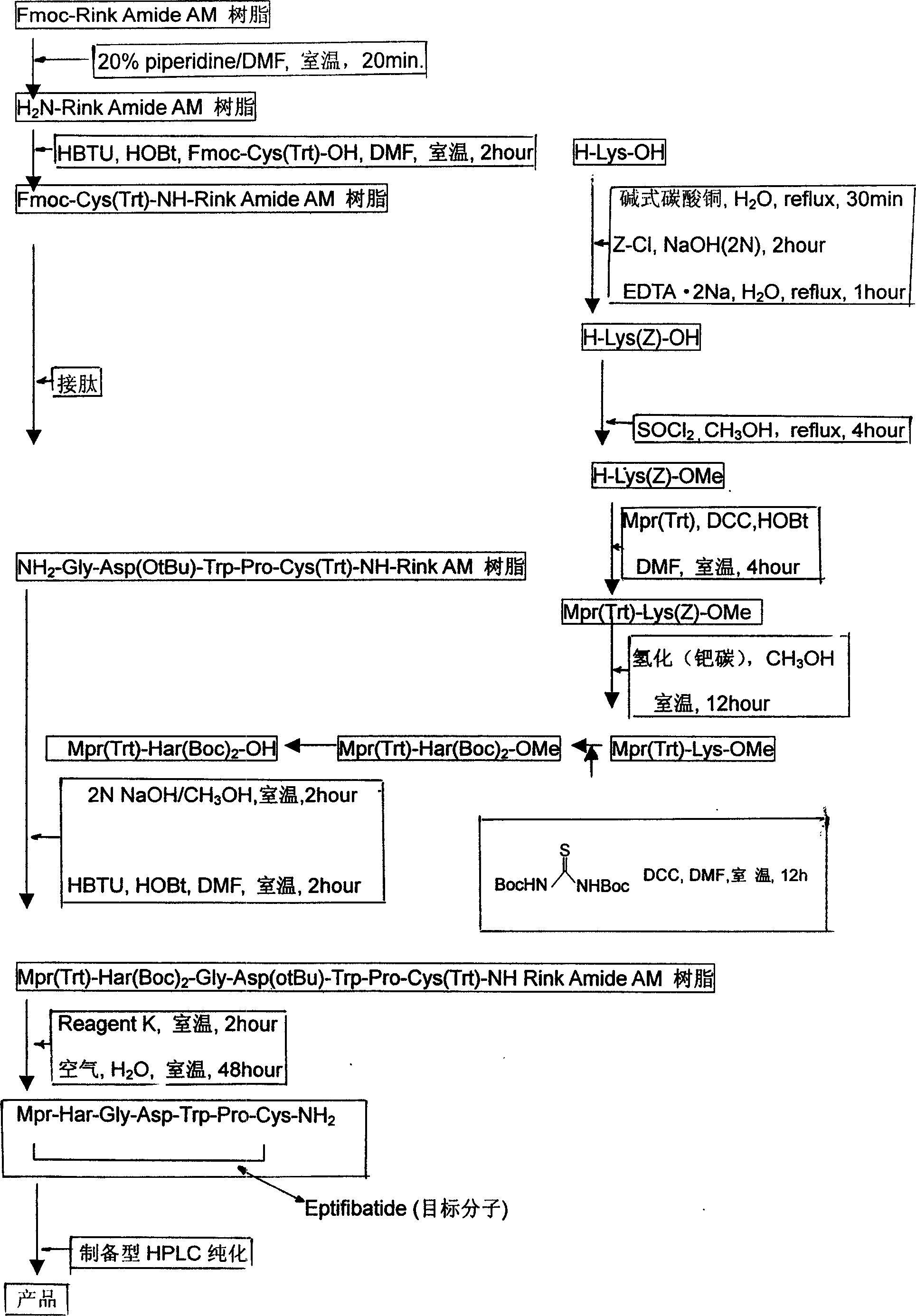
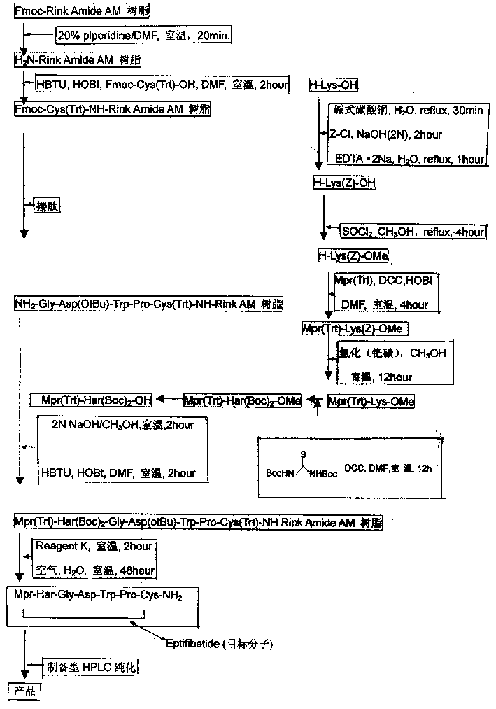
[0026] With reference to accompanying drawing, a kind of new process of preparing Efibuta, its preparation process step is:
[0027] a. Fmoc-Rink Amide AM resin in 20% piperidine, DMF, room temperature for 20 minutes, after de-Fmoc-protection to get H 2 N-Rink Amide AM resin;
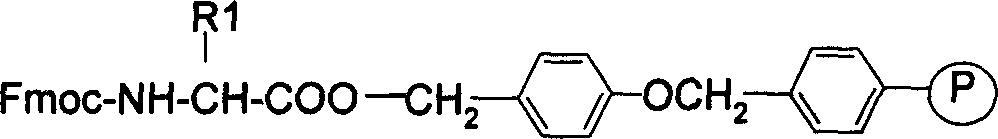
[0028] b. According to the method of solid phase synthesis in H 2 On the N-Rink Amide AM resin, the condensation reaction is carried out sequentially to connect the following protected amino acids: Fmoc-Cys(Trt)-OH; Fmoc-Pro-OH; Fmoc-Trp(Boc)-OH; Fmoc-Asp(tBu)-OH; Fmoc -Gly-OH; (not shown in the figure) to obtain Fmoc-Cys(Trt)-NH-Rink Amide AM resin and the resin connected to the subsequent protected amino acid, the selected condensing agent is HBTU, HOBt, DMF, and the reaction time is 2 hours at room temperature .
[0029] c. In the meantime, use piperidine to remove the Fmoc-protecting group to obtain NH 2 -Gly-Asp(OtBu)-Trp-Pro-Cys(Trt)-NH-Rink AM resin, using Kaiser test to detect the reaction p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com