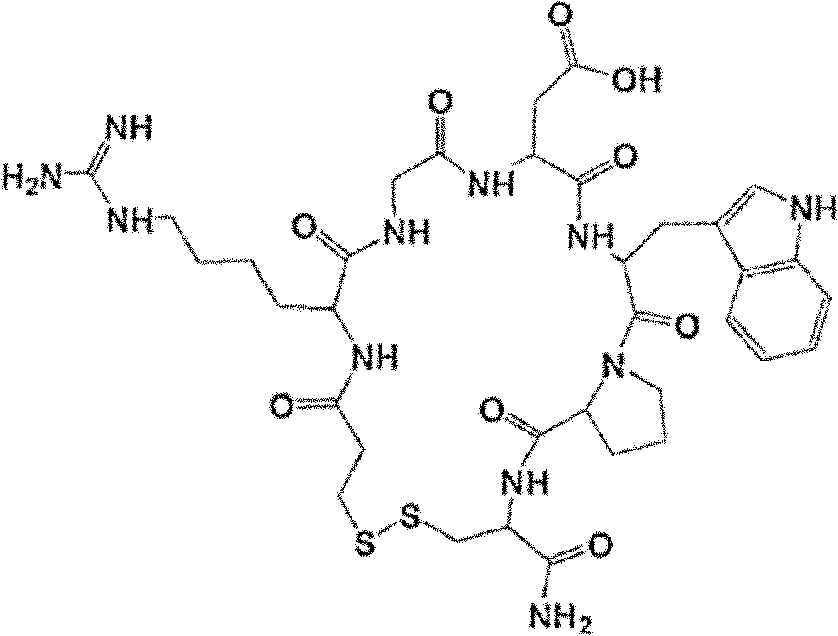
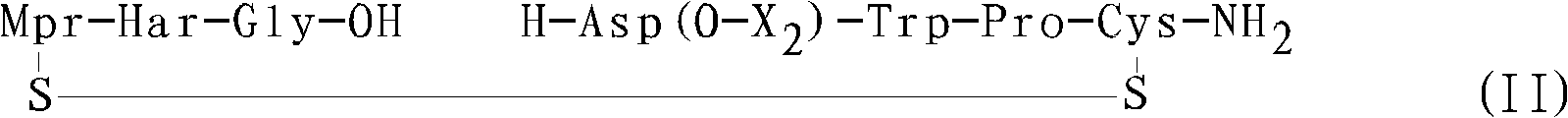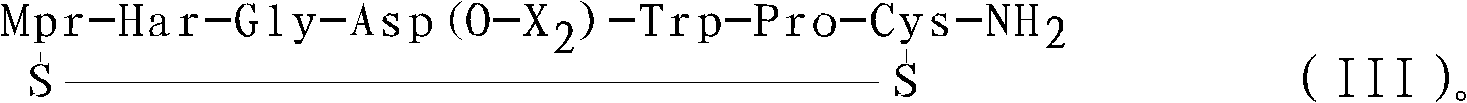Method for preparing eptifibatide and precursor thereof
A technology for eptifibatide and compounds, applied in the field of compound synthesis, can solve the problems of low purification yield, increased purification difficulty, waste of 6-peptide fragments, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] Embodiment 1: the synthesis of Boc-Asp(OtBu)-Trp-Pro-OH
[0061] In a 50 ml round bottom flask, add Boc-Asp(OtBu)-OSu (1.932g), H-Trp-Pro-NH 2 (1.506g), dissolved with 40 ml of anhydrous DMF, cooled to a constant temperature in an ice-water bath, added DCC (1.030g), stirred at room temperature for 3 hours, and detected that the reaction was complete. The precipitate produced by the reaction was removed by suction filtration, concentrated under reduced pressure to remove DMF, and then dissolved with a large amount of ethyl acetate, and then dissolved with NaHCO 3 Wash, wash with dilute hydrochloric acid, wash with saturated brine, dry with anhydrous sodium sulfate, and spin dry ethyl acetate to obtain a solid.
[0062] HPLC measures the purity of Boc-Asp(OtBu)-Trp-Pro-OH: greater than 93%, target content 2.648g, yield is 92.5%, mass spectrometry detects MS=572 (M + ).
Embodiment 2
[0063] Example 2: Boc-Asp(OtBu)-Trp-Pro-Cys(Npys)-NH 2 Synthesis
[0064] Add Boc-Asp(OtBu)-Trp-Pro-OH (2.362g), H-Cys(Npys)-NH 2 (1.372g), HOSu (0.575g), dissolved with 40 ml of anhydrous DMF, after adding DCC (1.030g) under ice-water bath, stirred at room temperature for 3 hours, and detected that the reaction was complete. The precipitate produced by the reaction was removed by suction filtration, concentrated under reduced pressure to remove DMF, and then dissolved with a large amount of ethyl acetate, and then dissolved with NaHCO 3 Wash, wash with dilute hydrochloric acid, wash with saturated brine, dry over anhydrous sodium sulfate, and evaporate to dry ethyl acetate to obtain a solid.
[0065] Determination of Boc-Asp(OtBu)-Trp-Pro-Cys(Npys)-NH by HPLC 2 Purity: greater than 91%, target content 3.823g, yield is 90.5%, mass spectrometry detects MS=845 (M + ).
Embodiment 3
[0066] Example 3: Synthesis of Boc-Har-Gly-Ome
[0067] In a 50 ml round bottom flask, add Boc-Har-OH (1.440g), NH2-Gly-Ome (0.445g), HOSU (0.575g), dissolve with 40 ml of anhydrous DMF, add DCC (1.030 g) under ice-water bath g) After stirring at room temperature for 2 hours, it was detected that the reaction was complete. The precipitate produced by the reaction was removed by suction filtration, concentrated under reduced pressure to remove DMF, and then dissolved with a large amount of ethyl acetate, and then dissolved with NaHCO 3 Wash, wash with dilute hydrochloric acid, wash with saturated brine, dry with anhydrous sodium sulfate, and spin dry ethyl acetate to obtain a solid.
[0068] HPLC measures the Boc-Har-Gly-Ome purity: greater than 94%, target object content 1.670g, yield is 93%, mass spectrometry detects MS=359 (M + ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com