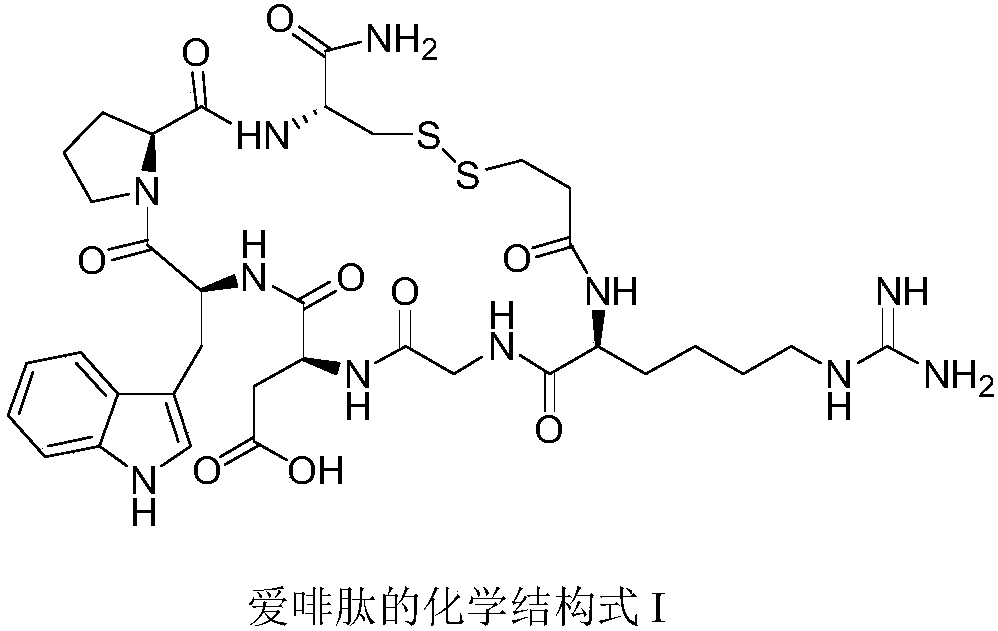
Preparation method for eptifibatide anticoagulant drug
A technology of anticoagulant and eptifibatide, which is applied in the field of preparation of anticoagulant drugs of eptifibatide, can solve the problems that the product purity is difficult to meet the ideal requirements, adverse drug reactions, and many synthesis steps, etc., so as to achieve easy removal and easy operation The effect of simplicity and low process cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Preparation of PNZ-Lys(Boc)-Gly-OBu-t
[0021] Add H-Gly-OBu-t (131g, 1mol) into the reaction flask, dissolve in 500mL of dichloromethane, and slowly add 220mL of DIEA (1.2mol) under ice cooling; the peptide condensation agent A (470g, 1.0mol ) and PNZ-Lys(Boc)-OH (425g, 1mol) were dissolved in 1000mL of dichloromethane, activated at 0°C for 10 minutes, and then slowly added dropwise to the above-mentioned H-Gly-OBu-t in dichloromethane solution , stirred at 0° C. for 30 minutes, and the reaction was monitored by TLC to complete. After the reaction solution was filtered, washed with aqueous sodium bicarbonate solution, aqueous hydrochloric acid and distilled water, the organic phase of dichloromethane was dried over anhydrous sodium sulfate, concentrated under reduced pressure, refined with ethyl acetate and chloroform to obtain a white solid PNZ-Lys(Boc)- Gly-OBu-t, yield 97.8%, purity 98.9%, MS[M+1] + 539.
Embodiment 2
[0023] Preparation of H-Lys(Boc)-Gly-OBu-t
[0024] Dissolve PNZ-Lys(Boc)-Gly-OBu-t (538g, 1mol) in 1000mL methanol, then add palladium carbon (5wt% palladium, 24g) / hydrogen, react at room temperature, filter after the reaction, and concentrate to a system of 200mL, cooled at 0°C and filtered to obtain solid H-Lys(Boc)-Gly-OBu-t, the yield was 98.8%, the purity was 98.7%, MS[M+1] + 360.
Embodiment 3
[0026] Preparation of H-Lys(Boc)-Gly-OBu-t
[0027] Dissolve PNZ-Lys(Boc)-Gly-OBu-t (538g, 1mol) in 1000mL methanol, then add platinum carbon (5wt% palladium, 24g) / hydrogen, react at room temperature, filter after the reaction, and concentrate to a system of 200mL, cooled at 0°C and filtered to obtain solid H-Lys(Boc)-Gly-OBu-t, the yield was 98.0%, the purity was 98.0%, MS[M+1] + 360.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com