Eptifibatide lyophilized powder injection with superior rehydration
A technology of freeze-dried powder injection and eptifibatide, which is applied in the directions of freeze-dried delivery, cyclic peptide components, powder delivery, etc., can solve the problems of increased dissolution rate, easy aggregation, eptifibatide adhesion, etc., and achieves good rehydration. , the effect of easy storage and transportation, excellent clinical medication compliance
Inactive Publication Date: 2011-10-05
杨毅跃
View PDF1 Cites 2 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
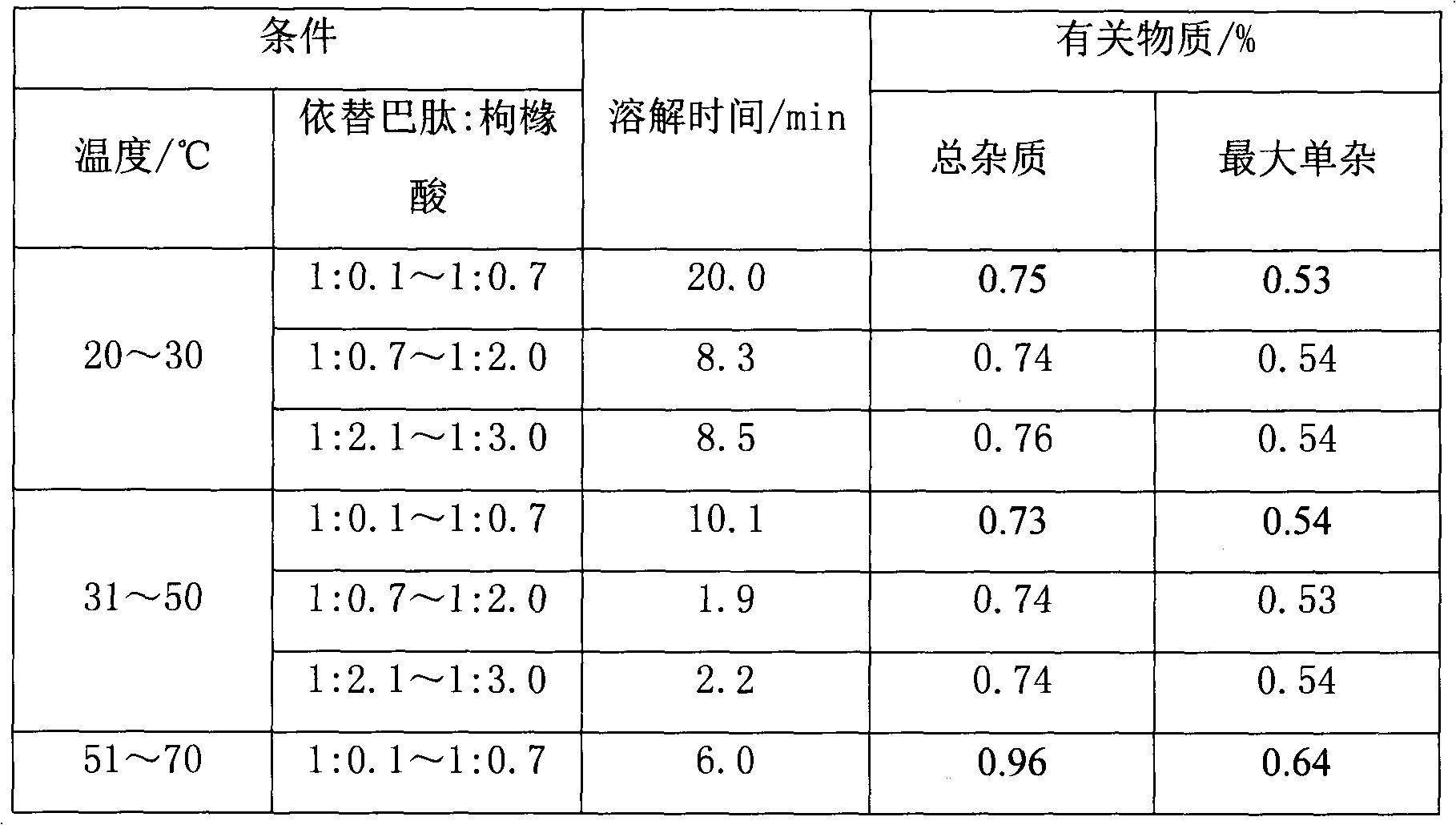
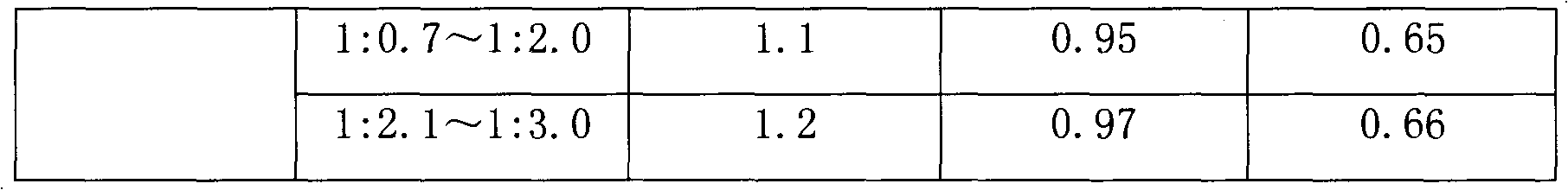
The dissolution rate of eptifibatide in a certain concentration of citric acid solution will greatly increase, which solves the problem of adhesion and easy aggregation of eptifibatide when it encounters water, and improves the operability of industrial production
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment 1
[0033] Example 1 20mg
[0034]
[0035]
Embodiment 2
[0036] Example 2 20mg
[0037]
Embodiment 3
[0038] Example 3 75mg
[0039]
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
The invention belongs to the field of pharmaceutical preparations, and relates to an eptifibatide lyophilized powder injection formula and preparation thereof. The eptifibatide lyophilized powder injection is composed of eptifibatide and pharmaceutically acceptable auxiliary materials, contains a given amount of citric acid, excipient, acidity regulator and water for injection, and is prepared by preparing the drug and the auxiliary materials into a solution by the water for injection, and performing a lyophilizing process (the water for injection is removed in the process). The eptifibatide lyophilized powder injection provided by the invention solves the problems of high viscosity, long lyophilizing process and poor rehydration of a high dose of polypeptide drug solution, and provides convenience for clinical medication.
Description
technical field [0001] The invention belongs to the field of pharmaceutical preparations, and relates to a formula of eptifibatide freeze-dried powder injection and a preparation process thereof. Background technique [0002] Every year, cardiovascular and cerebrovascular diseases such as cerebral thrombosis, cerebral infarction, myocardial infarction, coronary heart disease and arteriosclerosis claim the lives of 12 million people worldwide, which is close to a quarter of the total death toll in the world. The number of people who die from cardiovascular and cerebrovascular diseases in my country reaches more than 2.6 million every year, and 75% of the surviving patients are disabled, and more than 40% of them are severely disabled. According to the latest report from Business Communications, the blood disease treatment market, including thrombosis diseases, is growing rapidly in recent years. [0003] Antiplatelet therapy is an important part of anti-myocardial ischemia t...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More Patent Type & Authority Applications(China)
IPC IPC(8): A61K9/19A61K38/12A61K47/12A61K47/10A61P7/02A61P9/10
Inventor 杨毅跃
Owner 杨毅跃
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com