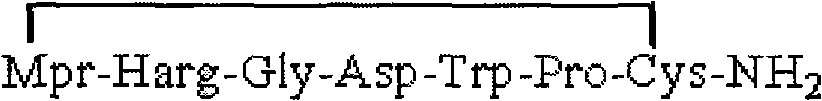Method for preparing Eptifibatide with solid phase method
A technology of solid-phase method and solid-phase oxidation, which is applied in the preparation method of peptides, chemical instruments and methods, blood diseases, etc., can solve the problems of low application value, complicated operation and production cycle, and achieve low cost and considerable economy Practical value, less environmental pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
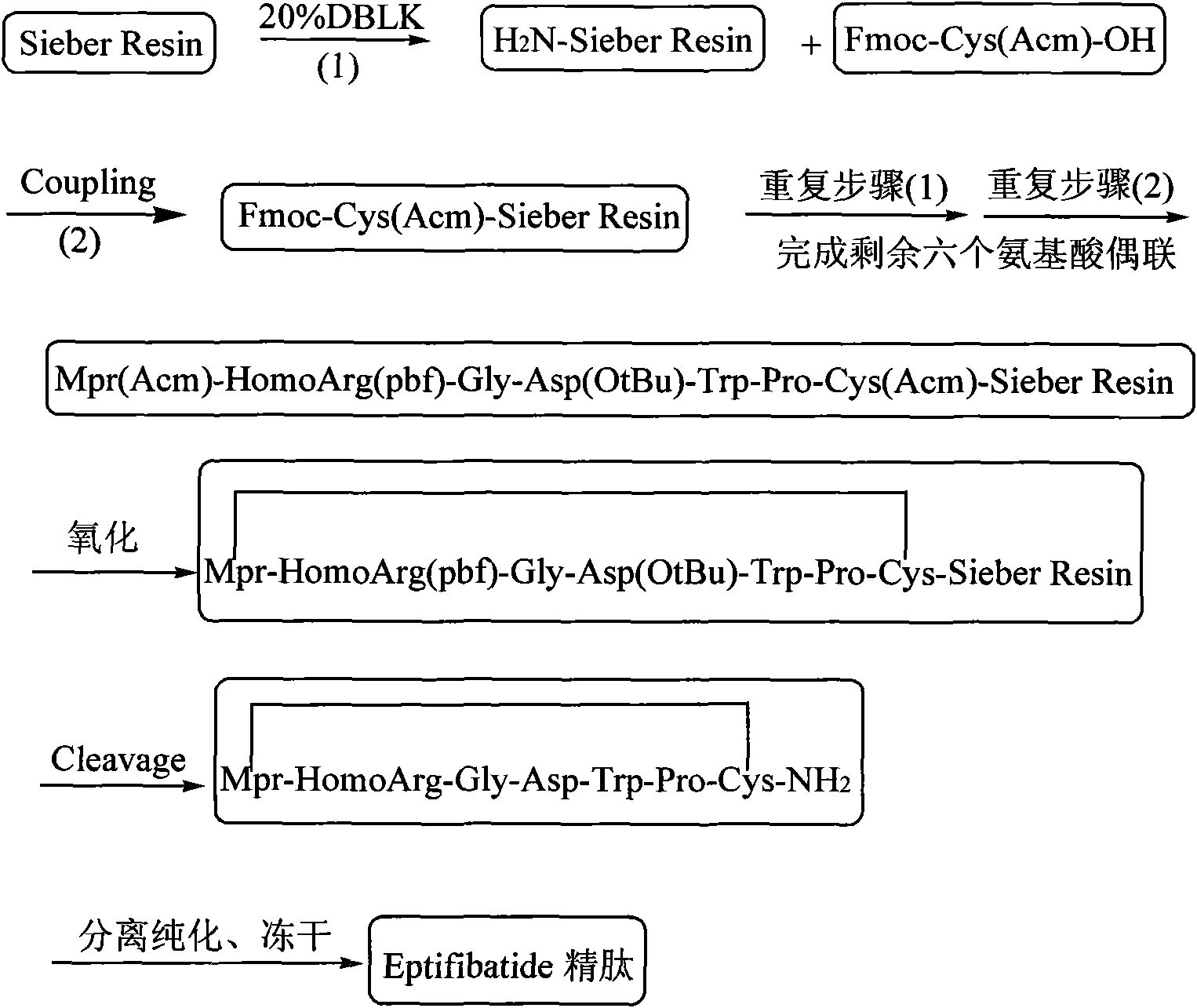
[0036] Example 1 Preparation of Eptifibatide peptide resin with full protection of linear side chains
[0037]Add 100.0 g of Sieber resin with a degree of substitution of 0.6 mmol / g to the solid phase reaction column, and swell the resin with DMF for 30 minutes. After the swelling is complete, use 20% DBLK to remove Fmoc for 10min+20min, and then wash with DMF six times. Fmoc-Cys(Acm)-OH 49.74g, HBTU 38.52g, HOBt16.2g and DIPEA 41.8ml were added to the reaction column, and the reaction was completed in two hours. Repeat the previous operation to perform the evening of Fmoc-Pro-OH, Fmoc-Trp-OH, Fmoc-Asp(OtBu)-OH, Fmoc-Gly-OH, Fmoc-Homoarg(pbf)-OH and Acm-Mpr-OH respectively. United. After the coupling was completed, the resin was washed three times with DMF, three times with DCM, three times with methanol, and dried in vacuum and weighed to obtain 172.1 g of a fully protected Eptifibatide peptide resin with linear side chains. Another preferred solution of this embodiment is: addin...
Embodiment 2
[0038] Example 2 Preparation of Eptifibatide peptide resin with full protection of oxidized side chain
[0039] 172.1 g of a fully protected Eptifibatide peptide resin with linear side chains was swollen with DMF for 30 minutes. After the swelling is complete, 304.8 g of iodine dissolved in an appropriate amount of DMF is added to the reaction column filled with resin, and the oxidation reaction is carried out for 2 hours. After the reaction was completed, it was washed ten times with DMF, three times with DCM, three times with methanol, and dried in vacuum and weighed to obtain 164.1 g of oxidized side chain fully protected Eptifibatide peptide resin.
Embodiment 3
[0040] Example 3 Preparation of Eptifibatide peptide resin with full protection of oxidized side chain
[0041] 1893 g of Eptifibatide peptide resin with fully protected linear side chains was swollen with DMF for 30 minutes. After the swelling is complete, 3350 g of iodine dissolved in an appropriate amount of DMF is added to the reaction column filled with resin, and the oxidation reaction is carried out for 3 hours. At the end of the reaction, it was washed ten times with DMF, three times with DCM, and three times with methanol. After drying in vacuum, it was weighed to obtain 1740 g of oxidized side chain fully protected Eptifibatide peptide resin.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com