Preparation method of eptifibatide
一种爱啡肽、爱啡肽精肽的技术,应用在多肽制备领域
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0029] The invention discloses a method for preparing eptifacin, and those skilled in the art can learn from the content of this article and appropriately improve the process parameters to realize it. In particular, it should be pointed out that all similar replacements and modifications are obvious to those skilled in the art, and they are all considered to be included in the present invention. The method and application of the present invention have been described through preferred embodiments, and the relevant personnel can obviously make changes or appropriate changes and combinations to the method and application described herein without departing from the content, spirit and scope of the present invention to realize and Apply the technology of the present invention.
[0030] The present invention provides a kind of preparation method of eptifibatide, comprising the following steps:
[0031] Step A: obtaining eptifatid fine peptide solution;
[0032] Step B: Take the ep...
Embodiment 1
[0042] The preparation of embodiment 1 eptifatid fine peptide solution
[0043] Add 100.0 g of Sieber resin (with a substitution degree of 0.6 mmol / g) into the solid-phase reaction column, and swell the resin with DMF for 30 minutes. After the swelling is complete, use 20% DBLK to remove Fmoc protection (10min+20min), and then wash with DMF six times.
[0044] Add Fmoc-Cys(Acm)-OH49.74g, HBTU38.52g, HOBt16.2g and DIPEA41.8mL into the above-mentioned solid-phase reaction column, and react for two hours to obtain Fmoc-Cys(Acm)-Sieber resin.
[0045] According to the method of coupling Fmoc-Cys(Acm)-OH mentioned above, Fmoc-Pro-OH, Fmoc-Trp-OH, Fmoc-Asp(OtBu)-OH, Fmoc-Asp(OtBu)-OH, Fmoc-Gly-OH, Fmoc-Homoarg(pbf)-OH and Acm-Mpr-OH were used for coupling. After the coupling was completed, the resin was washed three times with DMF, three times with DCM, shrunk three times with methanol, and weighed after vacuum drying to obtain 172.1 g linear side chain full-protected epthiptide r...
Embodiment 2
[0071] The preparation of embodiment 2 aptiferin
[0072] Take 5L of the eptifibatide fine peptide solution prepared in Example 1 and carry out rotary steaming. The temperature of the rotary steaming is 30°C. During the rotary steaming process, record the volume of the spin-out solution, and stop the rotary steaming when it reaches 3.2L. It takes 1.8 h, obtaining the concentrated solution of eptifatid peptide;
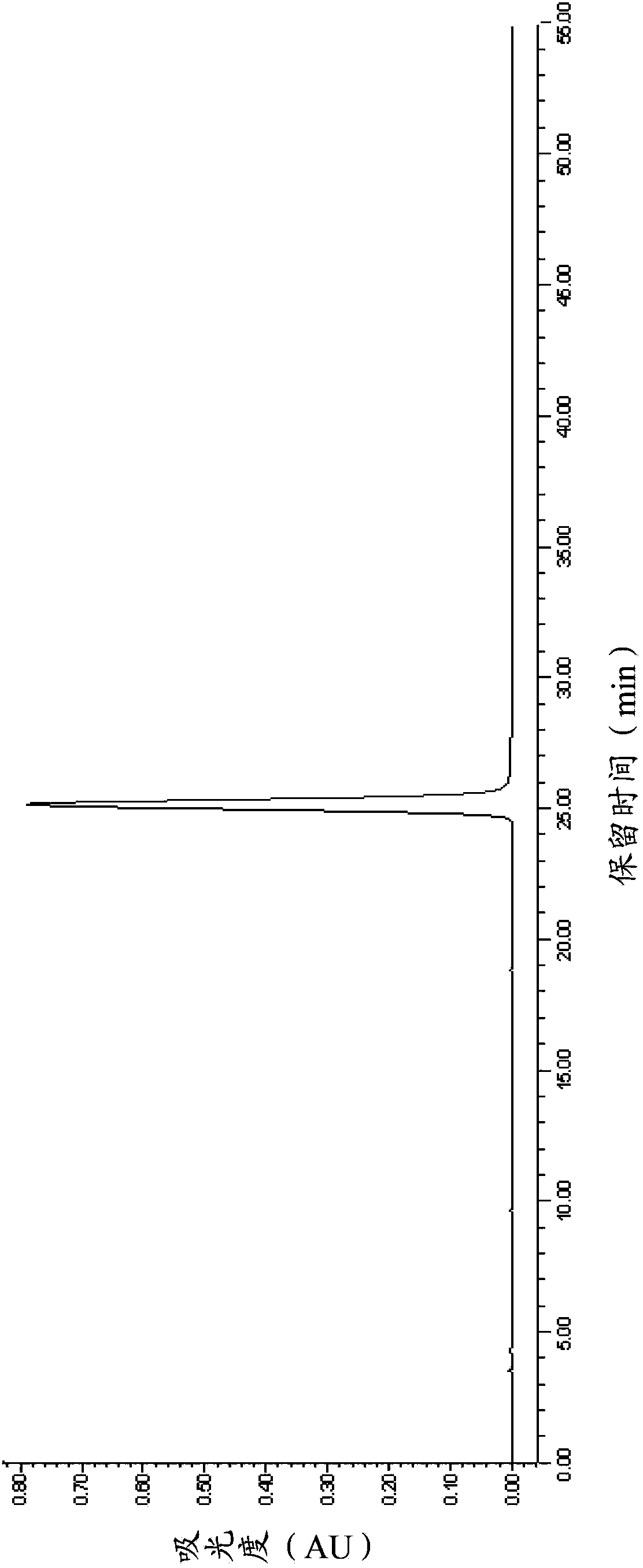
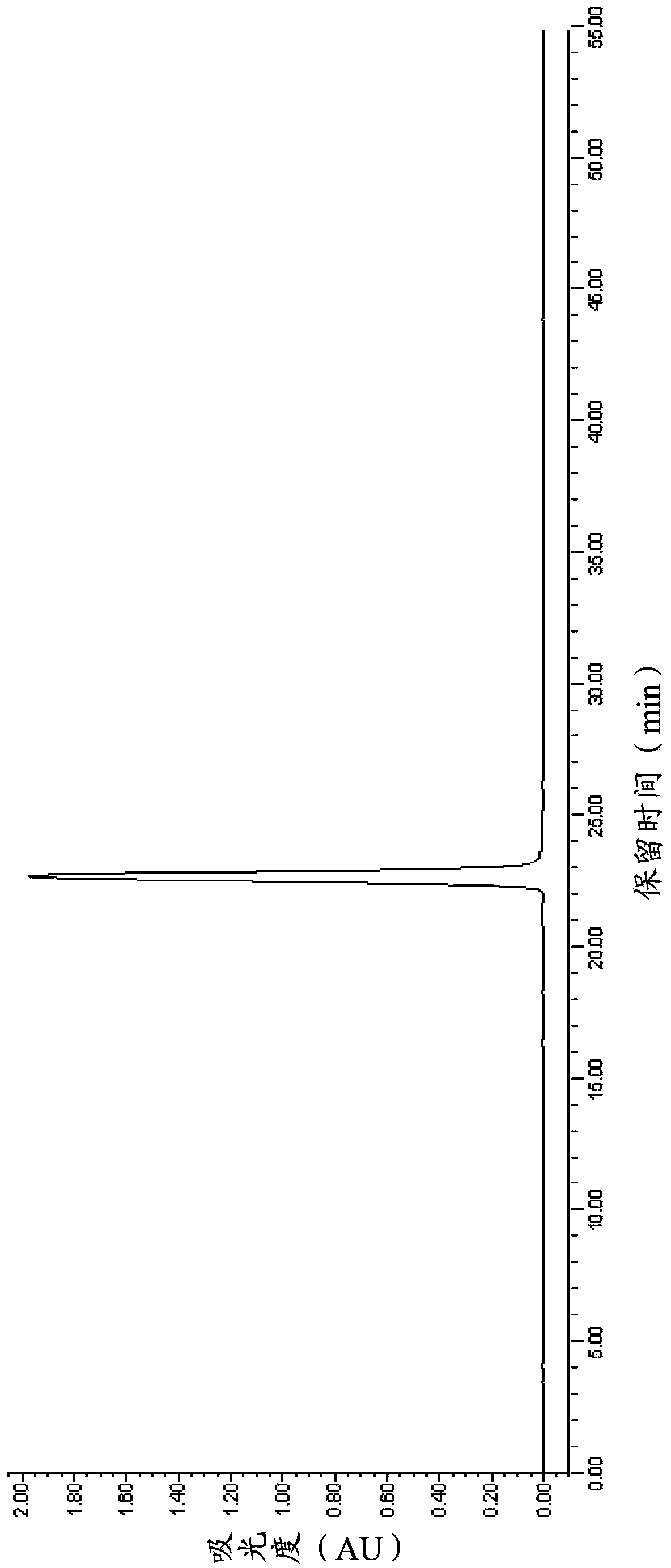
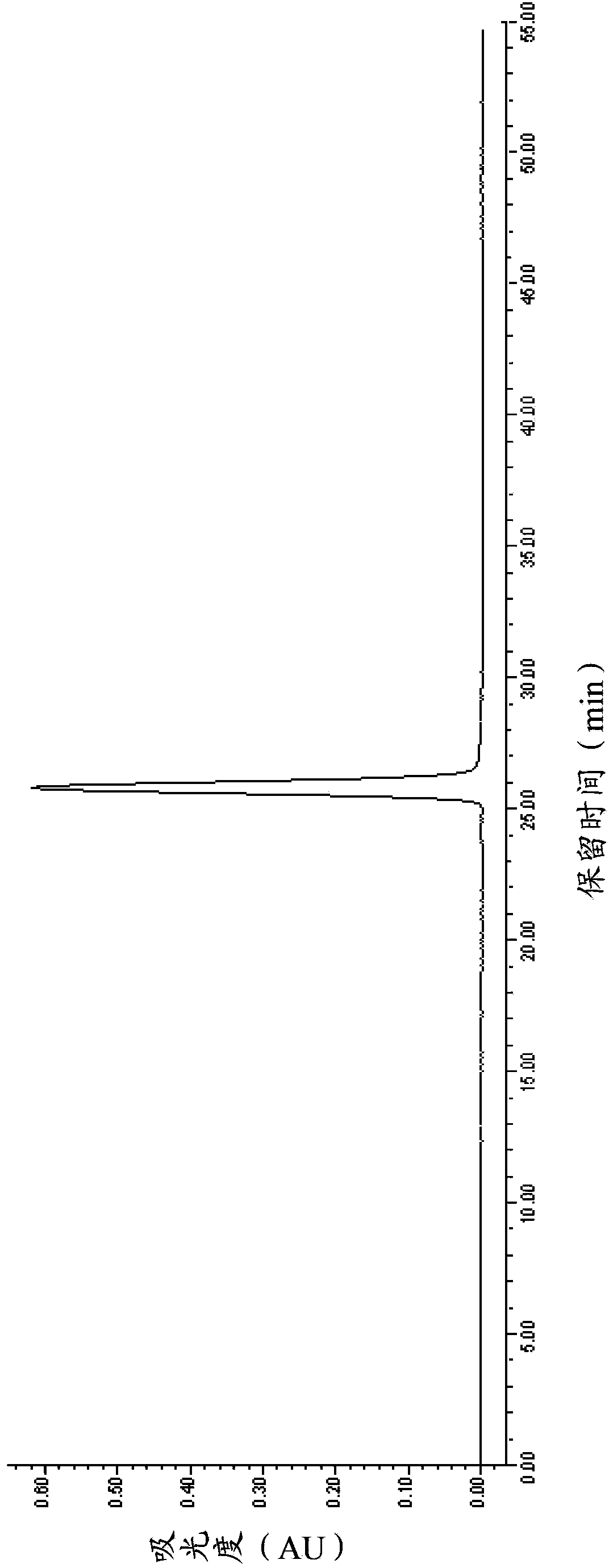
[0073] The purity, concentration and volume of the above-mentioned eptifibatide fine peptide concentrate were measured. After determination, the purity was 99.28% (of which the dimer impurity was 0.01%), the concentration was 15.3mg / mL, and the total volume was 1.8L. The HPLC spectrum is shown in figure 1 .
[0074] The above-mentioned concentrated solution of eptifatid peptide is still transparent at the end of the rotary evaporation, and no obvious precipitation occurs. 29.5 g of eptifibatide was obtained by freeze-drying the above-mentioned concentrated solution o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com