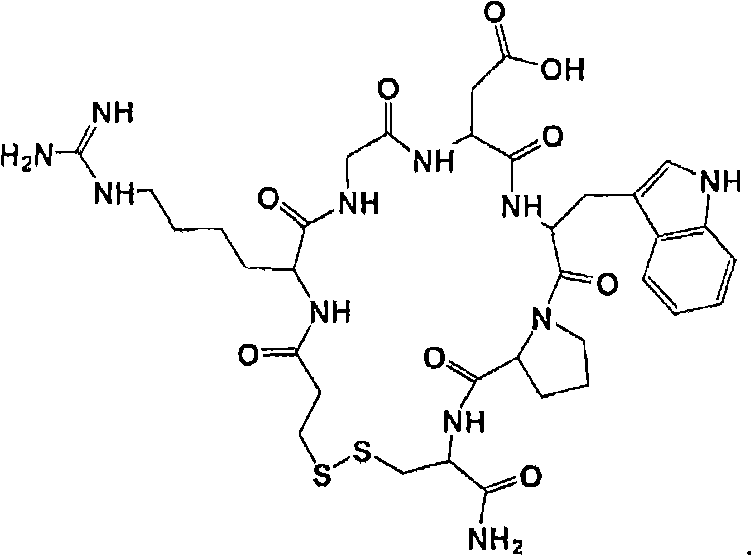
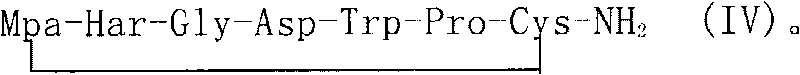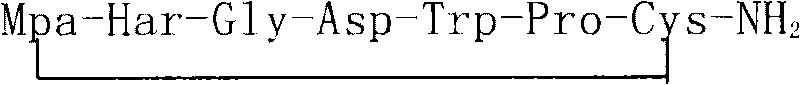Method for synthesizing eptifibatide
A technology of eptifibatide and synthesis method, which is applied in the preparation method of peptide, chemical equipment and method, peptide, etc., and can solve the problems of complex steps, many by-products of production, and difficulty in stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0100] Synthesis of Fmoe Rink amide AM resin (Fmoc-polypeptide resin)
[0101] The following substances Fmoc Rink linker / TBTU / HOBt / NMM (molar ratio 2.0:1.9:4) were condensed with AM resin (substitution degree range 0.8-1.0mmol / g), and stirred at room temperature for 3 hours.
[0102] The resin is used after washing and drying.
[0103] Fmoc deprotection
[0104] The Fmoc was removed by using 10% piperidine / 3% HOBT / 1% DBU / DMF solution, and the deprotection was performed twice consecutively, and the time was 10 min and 20 min respectively. Drain the deprotection solution and wash with DMF and methanol respectively. After exhaustion, Kaiser test was used to evaluate the removal of Fmoc.
[0105] Fmoc amino acid condensation steps:
[0106] The reactor was charged with Fmoc-AA-OH / HOBt (3 equiv / 3 equiv to Fmoc-Rink amide AM resin) / DMF solution followed by DIC (3 equiv to Fmoc-Rink amide AM resin). During the reaction for three hours, it was monitored by Kaiser test.
[0107] ...
Embodiment 2
[0123] Synthesis of Fmoc-Rink amide AM resin
[0124] The following substances Fmoc Rink linker / TBTU / HOBt / NMM (molar ratio 2.0:1.9:4) were condensed with AM resin (substitution degree range 0.8-1.0mmol / g), and stirred at room temperature for 3 hours.
[0125] and Ac20 / Pyridine / DMF (60v: 50v: 500v) capped to obtain. Drain the reaction solution thoroughly and wash 6 times. The resin is used after washing and drying.
[0126] Fmoc deprotection
[0127] The Fmoc was removed by using 20% piperidine / 1% HOBT / 0% DBU / DMF solution, and the deprotection was performed twice consecutively, and the time was 10 min and 20 min respectively. Drain the deprotection solution and wash with DMF and methanol respectively. After exhaustion, Kaiser test was used to evaluate the removal of Fmoc.
[0128] Fmoc amino acid condensation steps:
[0129] The reactor was charged with Fmoc-AA-OH / HOBt (1 equiv / 1 equiv to Fmoc-Rink amide AM resin) / DMF solution followed by DIC (1.5 equiv to Fmoc-Rink ami...
Embodiment 3
[0146] Synthesis of Fmoc-Rink amide AM resin
[0147] The following substances Fmoc Rink linker / TBTU / HOBt / NMM (molar ratio 2.0:1.9:4) were condensed with AM resin (substitution degree range 0.8-1.0mmol / g), and stirred at room temperature for 3 hours.
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of substitution | aaaaa | aaaaa |
| degree of substitution | aaaaa | aaaaa |
| degree of substitution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com