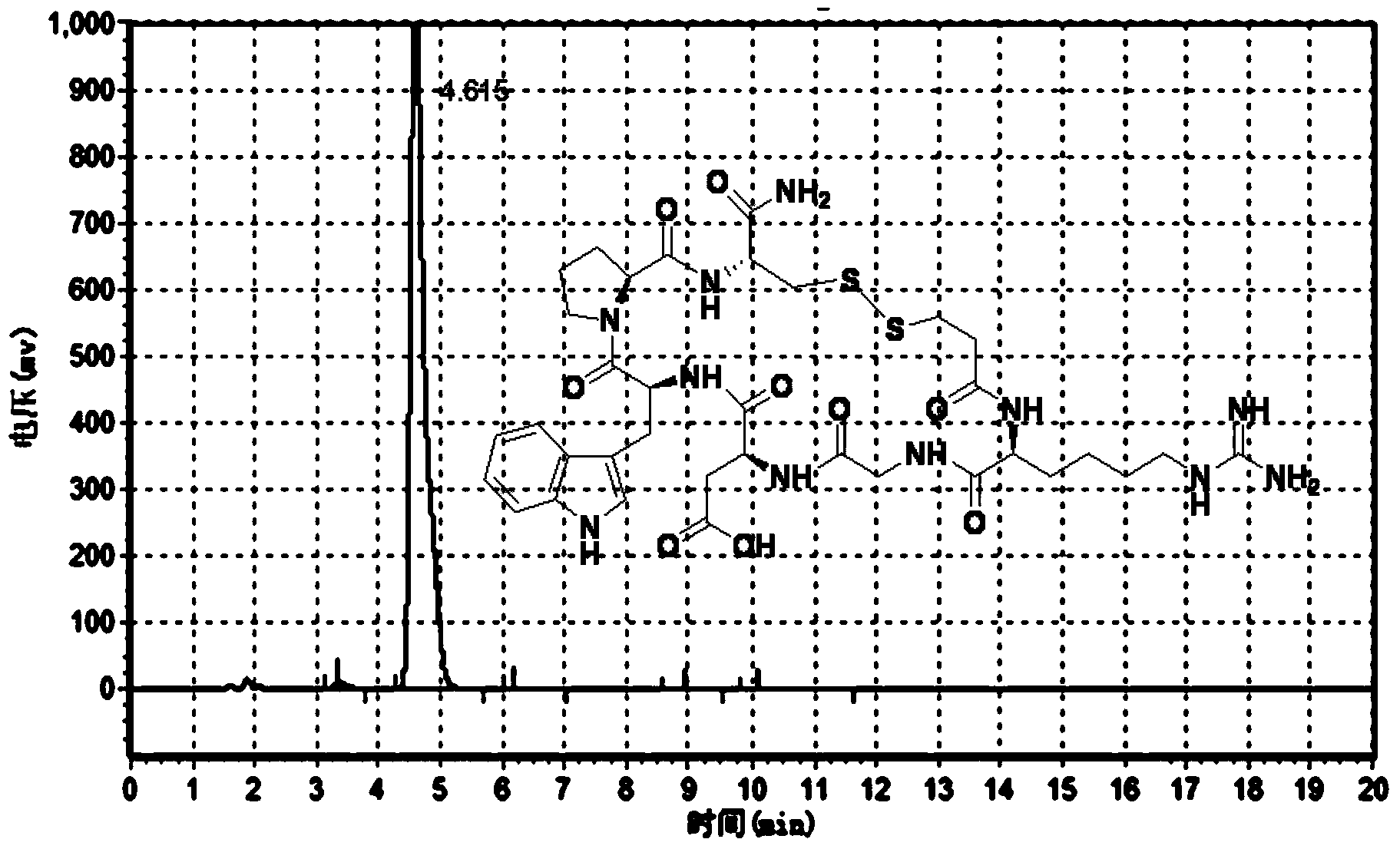
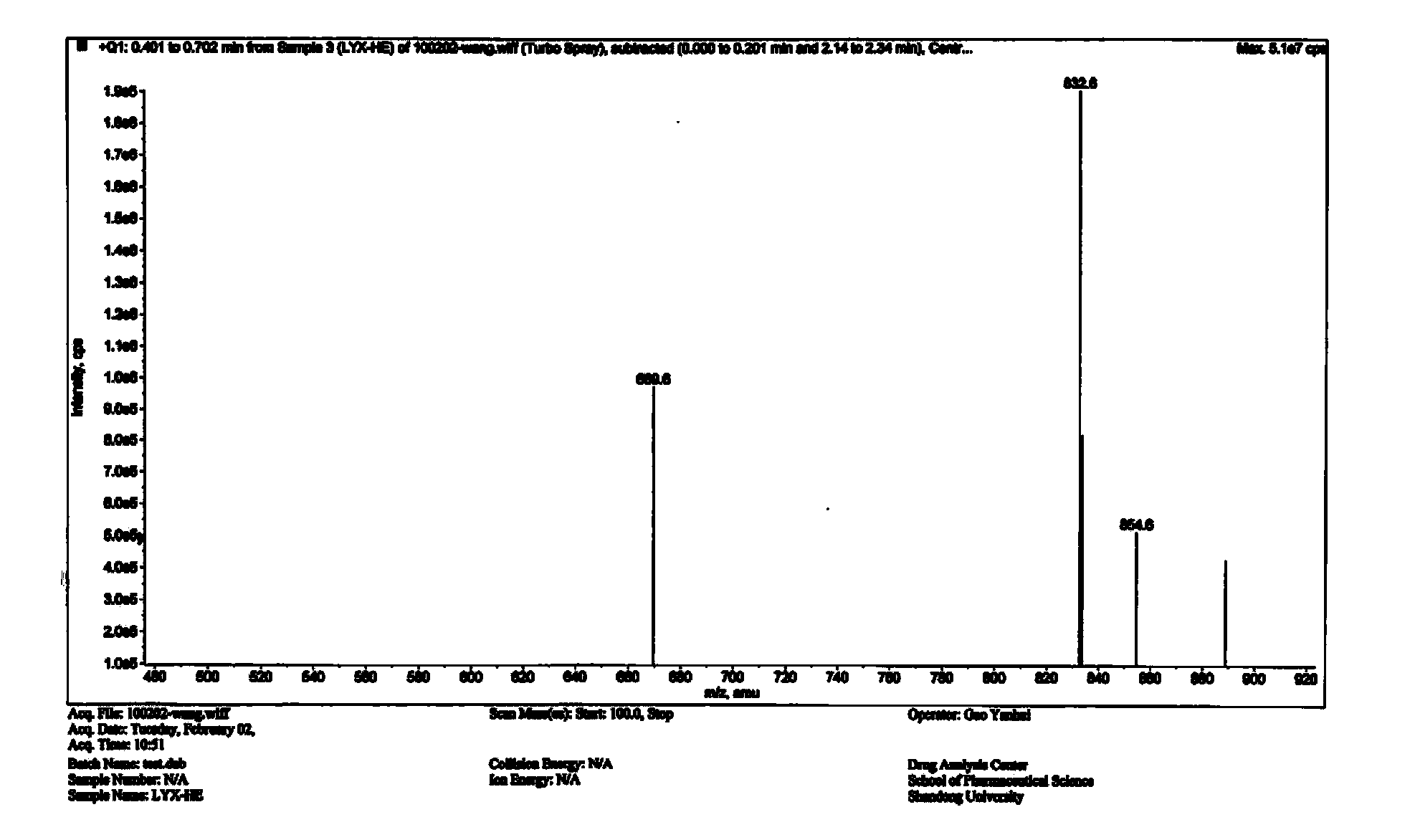
Liquid-phase synthesis method of eptifibatide
A liquid-phase synthesis, eptifibatide technology, applied in the field of biochemistry, can solve the problems of increased by-products, expensive raw materials, increased patient burden, etc., and achieves the effects of improving yield, reducing product cost, and improving production efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] Example 1. Fmoc-Pro-Cys(Trt)-NH 2 preparation of
[0059] H-Cys(Trt)-NH 2 (MW362.49, 1.812g, 5mmol) was dissolved in 10ml DCM, and DIEA (MW129.24, 1.1ml, 6mmol) was added under stirring; Fmoc-Pro-OH (MW337.37, 1.687g, 5mmol) and HOCt (MW .157.13, 0.943g, 6.0mmol) was dissolved in 25ml DCM, and DIC (MW126.20, 0.91ml, 6.0mmol) was slowly added dropwise under stirring to the above solution, and reacted for 15min. Then this mixed solution was added dropwise to H-Cys(Trt)-NH 2 / DIEA / DCM mixture, stirred at room temperature for 4 h, and TLC detected that the reaction was complete. The reaction solution was filtered with NaHCO 3 (0.2M, 25ml*2), hydrochloric acid (0.2M, 20ml*2) and saturated brine (30ml) were extracted sequentially, anhydrous Na 2 SO 4 After drying and concentrating under reduced pressure, an oily substance was obtained, and Et 2 O is placed, and a white solid is obtained, which is Fmoc-Pro-Cys(Trt)-NH 2 (MW.681.84), and separated and purified by HPLC (...
Embodiment 2
[0060] Example 2.H-Pro-Cys(Trt)-NH 2 preparation of
[0061] Take Fmoc-Pro-Cys(Trt)-NH 2 (MW681.84, 3.410g, 5mmol) dissolved in 50mlEt 2 In NH / DCM (10%), the reaction was carried out for 6h, and monitored by TLC until the reaction was complete. The reaction solution was decompressed at 30°C to remove DCM and Et 2 NH, the residue was dissolved in DCM and washed with hydrochloric acid (0.1M) until neutral, followed by Na 2 CO 3 (0.1M), water and saturated brine extraction, anhydrous Na 2 SO 4 After drying, concentrate under reduced pressure to obtain H-Pro-Cys(Trt)-NH 2 (MW.460.14), and separated and purified by HPLC (HPLC: 8.79min), product mass spectrometry analysis: MS=462.1[M+].
Embodiment 3
[0062] Example 3.Fmoc-Trp-Pro-Cys(Trt)-NH 2 preparation of
[0063] H-Pro-Cys(Trt)-NH 2 (MW459.60, 2.298g, 5mmol) was dissolved in 15ml DCM, and DIEA (MW129.24, 1.1ml, 6mmol) was added under stirring; Fmoc-Trp-OH (MW426.46, 2.132g, 5mmol) and HOCt (MW .157.13, 0.943g, 6.0mmol) was dissolved in 30ml DCM, and DIC (MW126.20, 0.91ml, 6.0mmol) was slowly added dropwise under stirring to the above solution, and reacted for 15min. Then this mixed solution was added dropwise to H-Pro-Cys(Trt)-NH 2 / DIEA / DCM mixture, stirred at room temperature for 6 h, and TLC detected that the reaction was complete. The reaction solution was filtered with NaHCO 3 (0.2M, 25ml*2), hydrochloric acid (0.2M, 20ml*2) and saturated brine (30ml) were extracted sequentially, anhydrous Na 2 SO 4 Dry and concentrate under reduced pressure to get an oil, add DCM / Et 2 O crystallized to obtain white solid Fmoc-Trp-Pro-Cys(Trt)-NH 2 (MW.868.05), and separated and purified by HPLC (HPLC: 11.63min), product m...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com