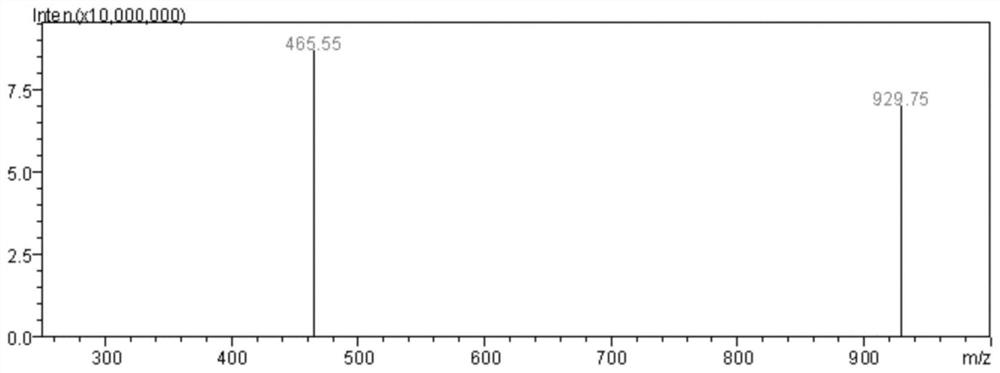
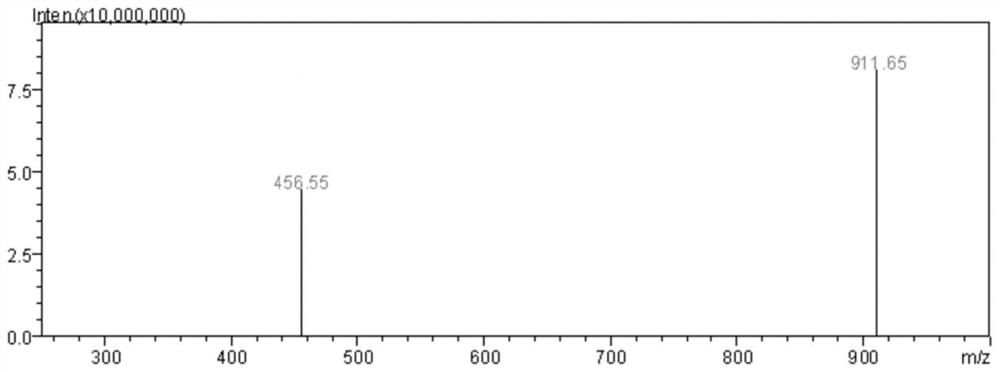
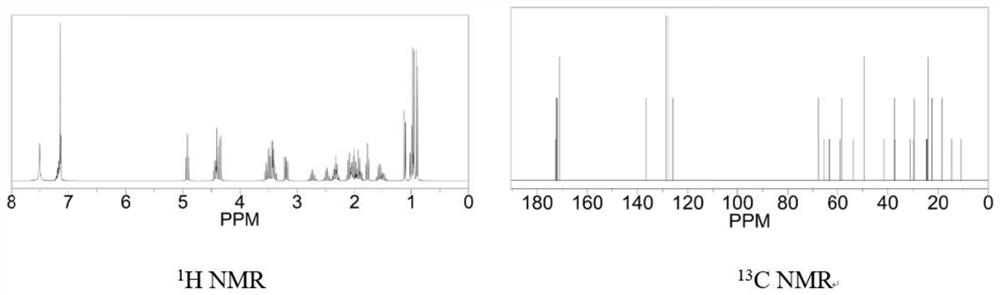
A kind of antitumor active peptide, synthesis method and application
A technology of anti-tumor activity and synthesis method, which is applied in the field of synthesis and anti-tumor active peptides, can solve the problems of poor anti-tumor activity, achieve excellent anti-tumor activity, reduce steric hindrance, and improve cyclization efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] The synthesis of the tumor suppressor active peptide C in this embodiment specifically includes the following steps:
[0061] (1) Coupling reaction between resin and amino acid
[0062] Weigh 2.00 g of 2-chlorotrityl chloride resin and put it into the sand core tube for polypeptide synthesis. Add DMF and use a rotary vane vacuum pump to blow and wash for 5-10 seconds, then use a circulating water-type multi-purpose vacuum pump to filter the liquid, add DCM to soak the resin and blow for 2-3 minutes to fully expand and then filter. Weigh 451.4 mg of Fmoc-Val-OH and put it into a sand core tube, add DCM as a reaction solvent, add 1 mL of DIEA dropwise as a condensation reagent, blow the reaction at room temperature for 30 minutes, pump out the liquid, add DMF to wash 4 times and filter with suction. Add DCM again, add dropwise 4mL of anhydrous methanol and 4mL DIEA to react for 30min, and cap the unreacted sites of the resin. After the reaction was finished, filter with...
Embodiment 2
[0082] This embodiment is an antitumor activity verification experiment for Samoamide A and Samoamide C, including the following steps:
[0083] (1) DPP-4 enzyme activity inhibition experiment
[0084] The imported DPP-4 enzyme inhibitor sitagliptin and the experimental method provided were used to verify the inhibitory effect of several derivatives on DPP-4 enzyme activity. Take an opaque 96-well black microtiter plate, and drop 1 μL of DPP-4 enzyme solution and 49 μL of test buffer into each control. Then set up each group of experiments:
[0085] Group 1-blank group: without adding any substance;
[0086] Group 2 - sample group to be tested: add 25 μL of peptide solution with DMSO as solvent, the concentration is 100 μg / mL, and test the experimental groups of Samoamide A and Samoamide C peptide solutions respectively;
[0087] Group 3-inhibitor comparison group: pipette 2 μL of the sitagliptin inhibitor mother solution provided in the kit, add buffer to dilute it 100 tim...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com