A method of purifying a peptide
A technology for cyclic peptides and purity, applied in the field of purified peptides
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
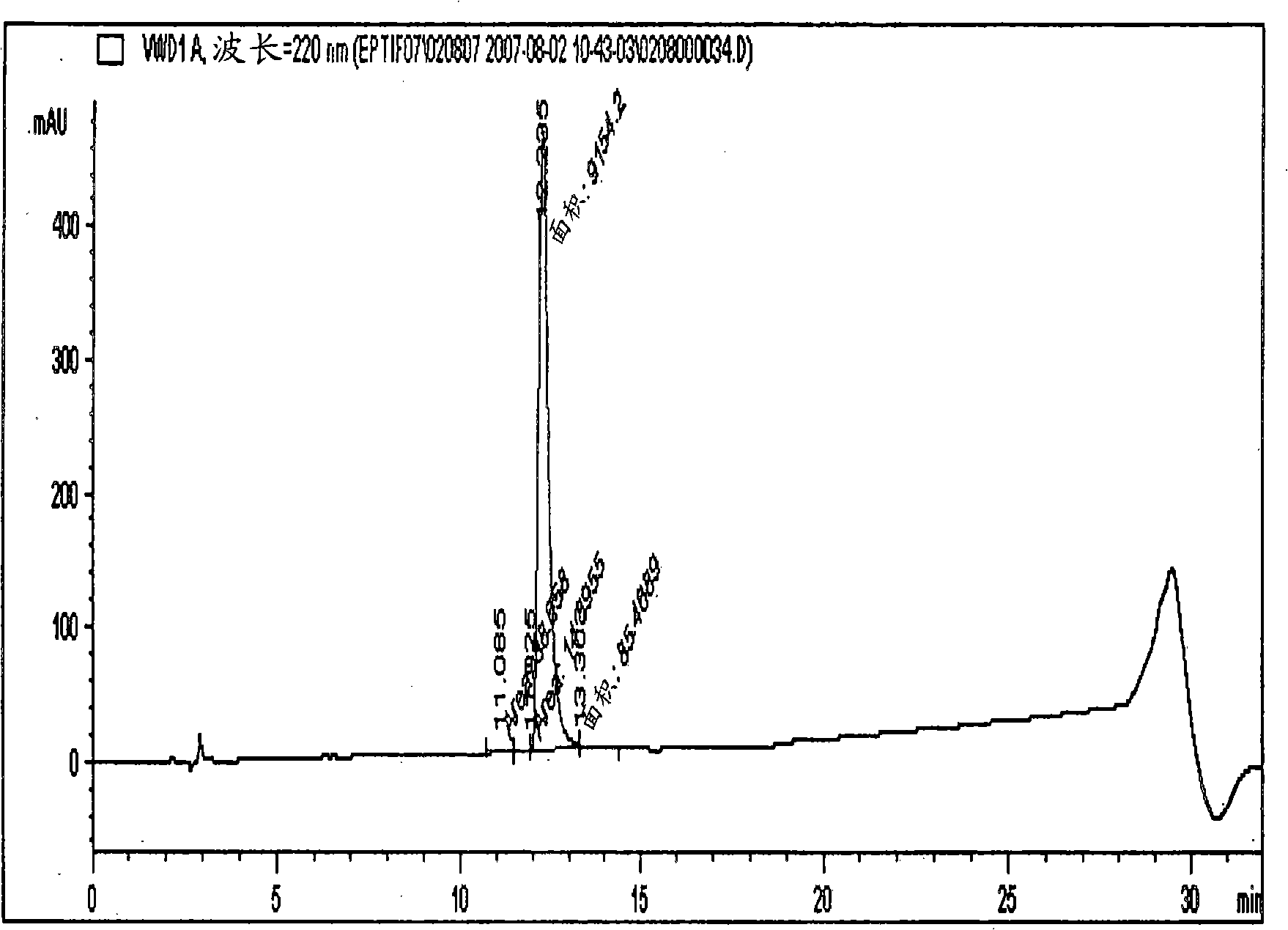
[0113] A 66% pure eptifibatide TFA salt prepared by solid-phase synthesis was used for purification on a polymer-based resin-packed column. Eptifibatide TFA salt was initially dissolved in a 1:1 mixture of acetonitrile and 50 mM acetic acid to obtain a clear solution. The obtained solution was further diluted to an acetonitrile concentration of 5% and an eptifibatide concentration of <2 g / L using 50 mM acetic acid. The solution was filtered and loaded onto the column.
[0114] Amberchrom HPR10 (particle size 10 μm and pore size ) resin-packed column. The filtered eptifibatide solution was loaded onto the column at a flow rate < 360 cm / h. Loading of the peptide on the column was done at <10 g / L concentration of resin. After loading, the column was rinsed with a lower percentage (5%) of acetonitrile in 50 mM acetic acid solution. Pure product was eluted from the column by performing a linear gradient of 8-14% of acetonitrile (buffer B) for 25 CV, while 50 mM acetic acid wa...
Embodiment 2
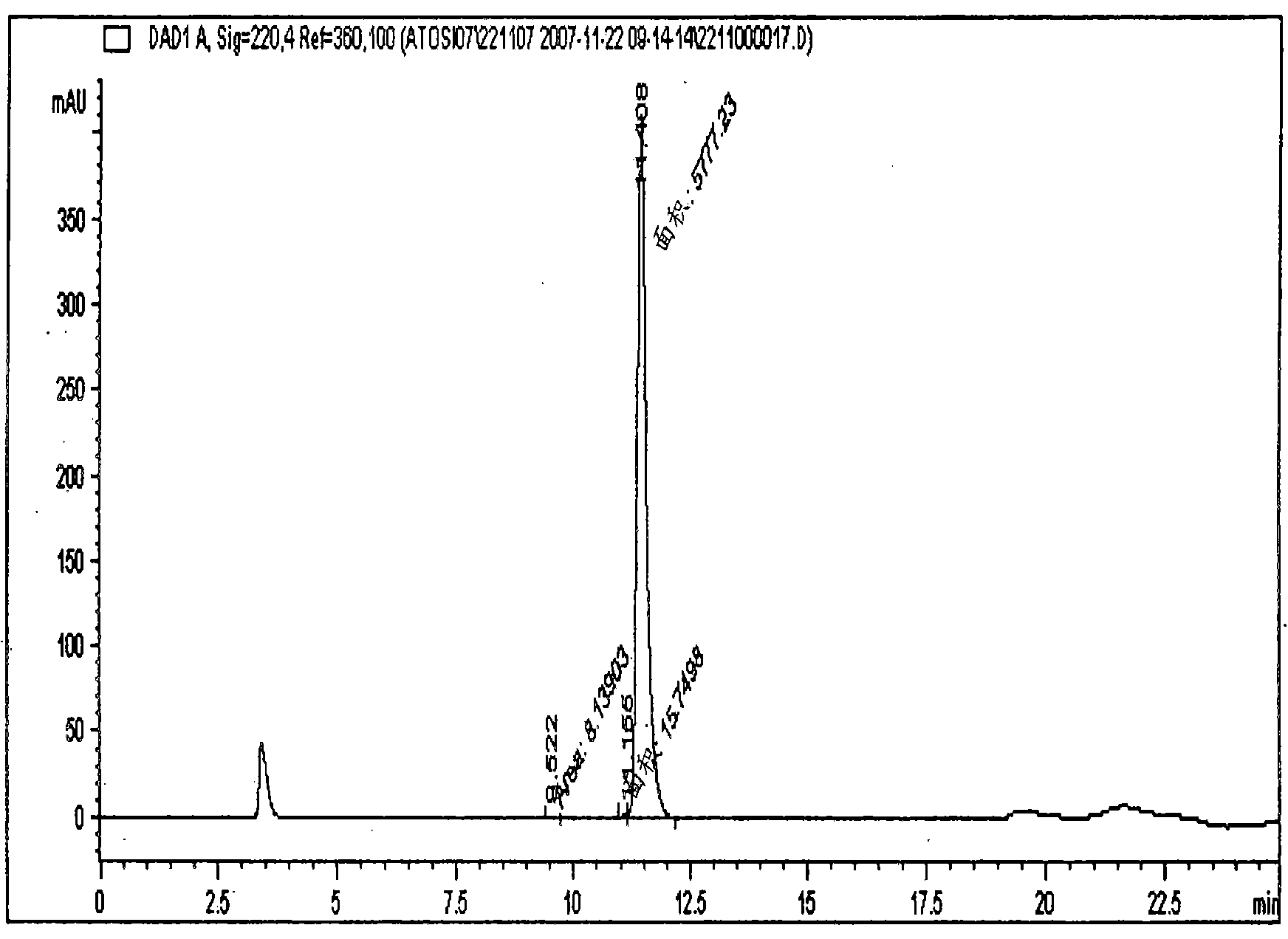
[0120] A 69.5% pure eptifibatide TFA salt prepared by solid phase synthesis was used for purification on a polymer-based resin packed column. Eptifibatide TFA salt was initially dissolved in a 1:1 mixture of acetonitrile and 50 mM acetic acid to obtain a clear solution. The obtained solution was further diluted to an acetonitrile concentration of 5% and an eptifibatide concentration of <2 g / L using 50 mM acetic acid. The solution was filtered and loaded onto the column. The pH of sodium acetate was adjusted to 3.0 with acetic acid.
[0121] Amberchrom HPR10 (particle size 10 μm and pore size ) resin-packed column. The filtered solution of eptifibatide was loaded onto the column at a flow rate < 360 cm / h. Loading of the peptide on the column was performed at a concentration < 10 g / L of the resin. After loading, the column was rinsed with a lower percentage (5%) of acetonitrile in 10 mM sodium acetate, pH 3.0. Pure product was eluted from the column by performing a 9-12% ...
Embodiment 3
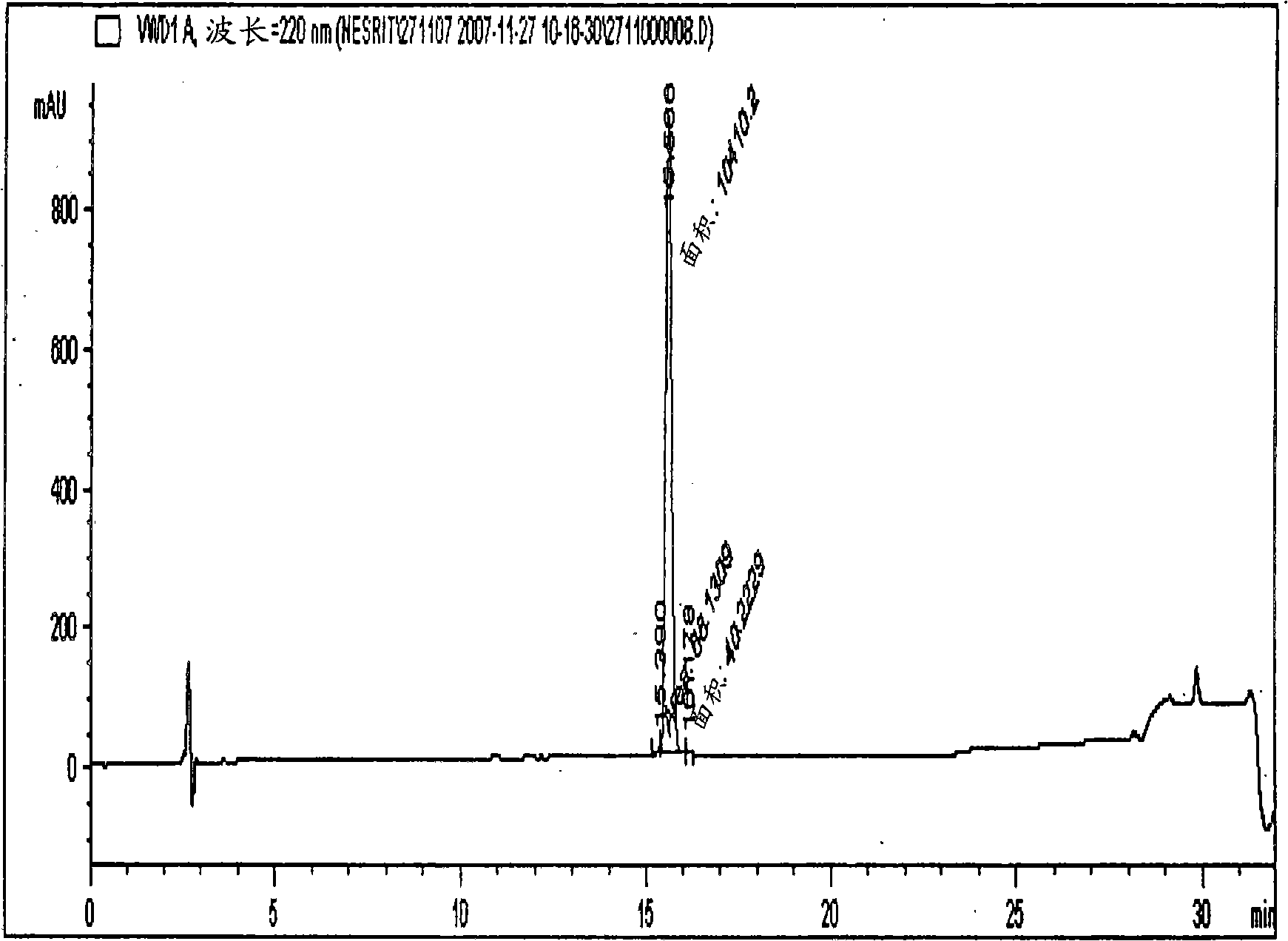
[0125] A 69.5% pure eptifibatide TFA salt prepared by solid phase synthesis was used for purification on a polymer-based resin packed column. Eptifibatide TFA salt was initially dissolved in a 1:1 mixture of acetonitrile and 50 mM acetic acid to obtain a clear solution. The obtained solution was further diluted to an acetonitrile concentration of 5% and an eptifibatide concentration of <2 g / L using 50 mM acetic acid. The solution was filtered and loaded onto the column.
[0126] Amberchrom HPR10 (particle size 10 μm and pore size ) resin-packed column. The filtered eptifibatide solution was loaded onto the column at a flow rate < 360 cm / h. Loading of the peptide on the column was performed at a concentration < 10 g / L of the resin. After loading, the column was rinsed with a lower percentage (5%) of acetonitrile in 10 mM citric acid, pH 2.5. Pure product was eluted from the column by performing a 9-12% linear gradient of acetonitrile (buffer B) for 25CV, while 10 mM citri...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com