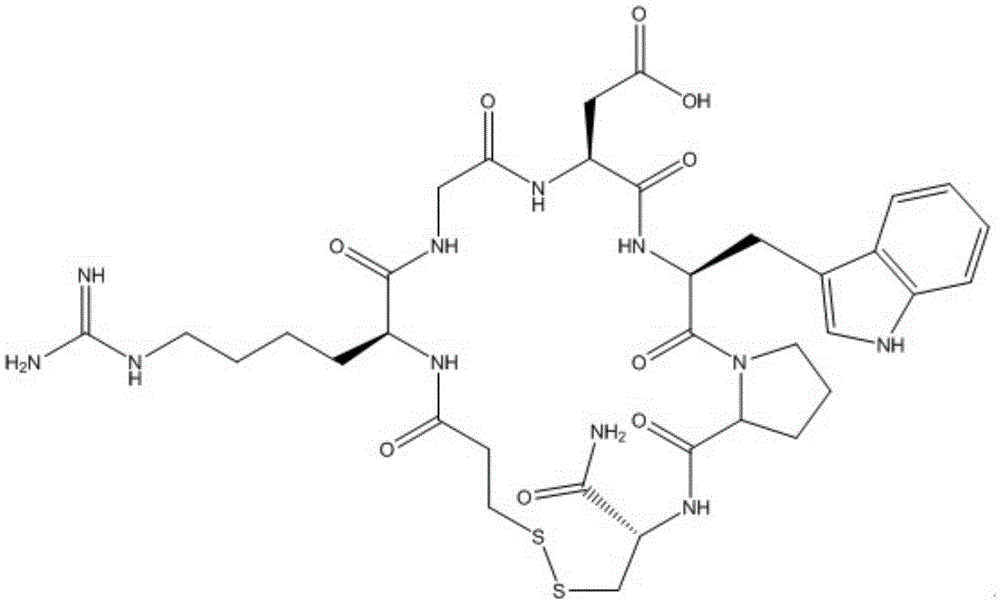
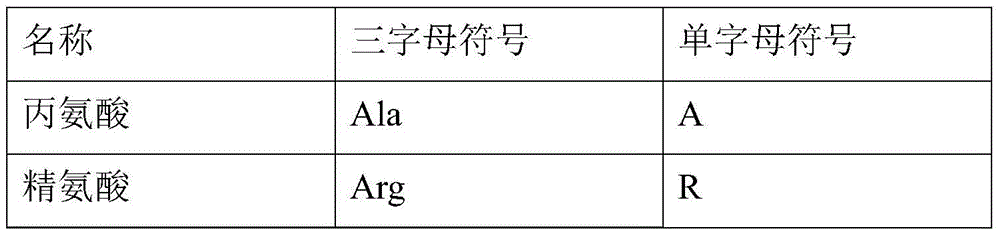
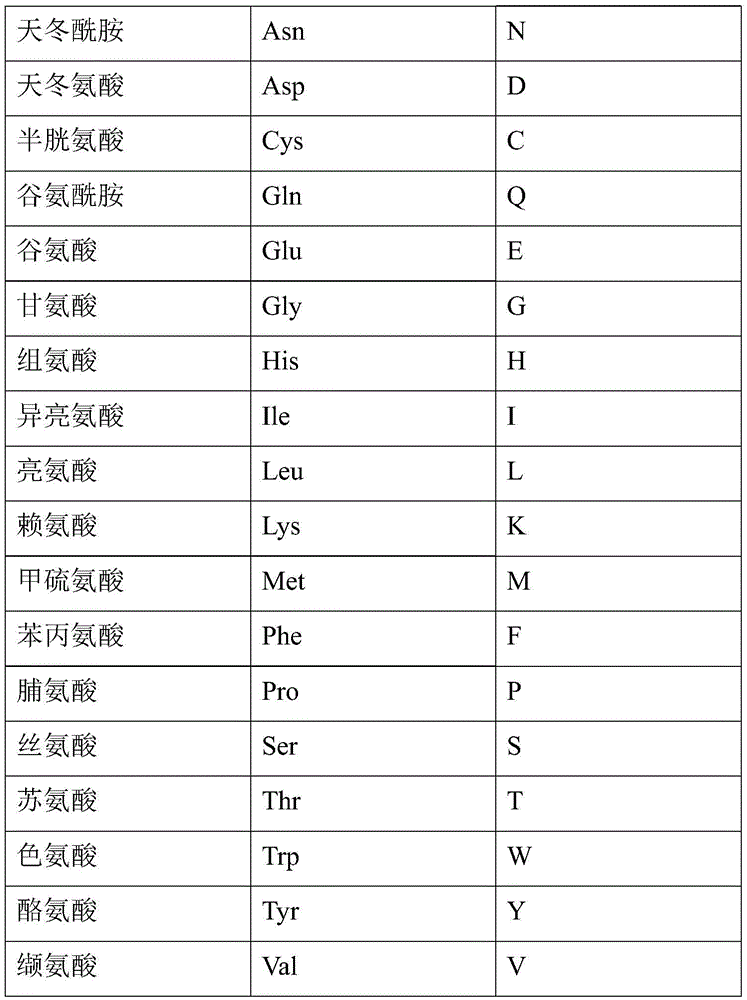
Eptifibatide synthesis method
A technology of eptifibatide and a synthesis method, which is applied in the field of eptifibatide synthesis, can solve the problems of affecting the purity of crude products, the reaction is not easy to be completed, and the purification difficulty is increased, and achieves shortening time, shortening production cycle, and cyclization reaction. quick effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] 1. Prepare Fmoc-Cys(Trt)-Linker-AM resin: wash 80mL Fmoc-Linker-AM resin with 10mLDMF twice (5mLDMF / time); then soak in 10-15mL dichloromethane for about 10 minutes to fully swell the resin , add 10-15mL of piperidine and DMF with a volume ratio of 1:4, heat in a water bath or oil bath or microwave, react at 60°C to 80°C for 2 minutes, drain, wash with 15mLDMF three times (5mLDMF / time) , add 10-15mL of the mixture of Fmoc-Cys(Trt)-OH, Oxyma and DIC dissolved in DMF, heat in a water bath, oil bath or microwave, react at 60°C to 80°C for about 15 minutes, drain, and then Washed three times with 15mLDMF (5mLDMF / time), the Fmoc-Cys(Trt)-Linker-AM resin was obtained.
[0041] Please note: Oxyma can also be replaced by HOBt. The amount of amino acid (such as Fmoc-Cys(Trt)–OH) and condensation reagent (such as Oxyma and DIC) used is twice or more than the mole of resin, and the following process is the same.
[0042] 2. To prepare Fmoc-Pro-Cys(Trt)-Linker-AM resin, after the ...
Embodiment 2
[0051] 1. Preparation of Fmoc-Cys(Trt)-Linker-AM resin: wash 60mL of Fmoc-Linker-AM resin with 12mLDMF twice (4mLDMF / time); then soak in 10mL of dichloromethane for about 10 minutes to fully swell the resin, add 15mL of piperidine and DMF with a volume ratio of 1:4, heated by microwave, reacted at 60-80°C for 2 minutes, drained, washed three times with 12mL of DMF (4mLDMF / time), added Fmoc-Cys dissolved in DMF (Trt)-OH, Oxyma and DIC mixture 15mL, heated by microwave, reacted at 60-80 ℃ for 15 minutes, sucked dry, and then washed three times with 12mLDMF (4mLDMF / time), Fmoc-Cys(Trt )-Linker-AM resin.
[0052] Please note: Oxyma can also be replaced by HOBt, the amount of amino acid (such as Fmoc-Cys(Trt)–OH) and condensation reagent (such as Oxyma and DIC) used is twice the mole of resin, the following process is the same.
[0053] 2. To prepare Fmoc-Pro-Cys(Trt)-Linker-AM resin, after the above steps, add 15mL of piperidine and DMF with a volume ratio of 1:4, heat with micro...
Embodiment 3
[0062] 1. Preparation of Fmoc-Cys(Trt)-Linker-AM resin: wash 60mL of Fmoc-Linker-AM resin with 9mLDMF twice (3mLDMF / time); then soak in 15mL of dichloromethane for about 10 minutes to fully swell the resin, add 10 mL of piperidine and DMF with a volume ratio of 1:3, heated in a water bath, reacted at 70°C for 3 minutes, drained, washed three times with 9 mL of DMF (3 mL of DMF each time), added Fmoc-Cys (Trt )-OH, Oxyma and DIC mixture 12mL, heated in a water bath, reacted at 70°C for 18 minutes, sucked dry, and then washed three times with 12mLDMF (4mLDMF / time) to obtain Fmoc-Cys(Trt)-Linker- AM resin.
[0063] Please note: Oxyma can also be replaced by HOBt, the amount of amino acid (such as Fmoc-Cys(Trt)–OH) and condensation reagent (such as Oxyma and DIC) used is twice the mole of resin, the following process is the same.
[0064] 2. To prepare Fmoc-Pro-Cys(Trt)-Linker-AM resin, after the above steps, add 10mL of piperidine and DMF with a volume ratio of 1:3, heat in a wa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com