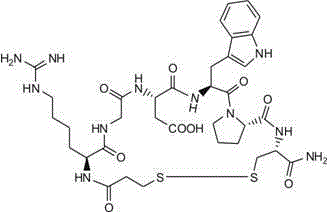
Eptifibatide preparing method
A technology of efibactide and efibactide, which is applied in the field of drug synthesis, can solve the problems of easy generation of impurities, complicated process, harsh acid hydrolysis conditions, etc., and achieve the effect of avoiding the generation of impurities
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Example 1: Synthesis of Mpr(Mmt)-Harg-OH.HCl
[0052] Dissolve 200g 3-mercaptopropionic acid (Mpr) with 2.5LN,N-dimethylformamide (DMF), cool in ice water, and slowly add 582g 4-methoxytriphenylchloromethane (Mmt-Cl) under stirring, add After 4-methoxytriphenylchloromethane, the reaction was stirred for another 8 hours. Add 5L of water to the reaction solution under stirring to separate out solids. Wash the solids with water 5 times (2L each time), filter, and then wash with ethyl acetate 3 times (2L each time), filter, and dry at 60°C to obtain 611g product Mpr(Mmt) -OH (85.7% yield).
[0053] Dissolve 600gMpr(Mmt)-OH and 201gHOSu with 3L tetrahydrofuran (THF), cool in ice water, and slowly add 327gN,N'-dicyclohexylcarbodiimide (DCC) with stirring, naturally warm to room temperature, and react at room temperature for 15 hour. Filter, wash the solid with THF 3 times (0.5L each time), combine the filtrate and washing liquid, concentrate at least the amount in vacuum at 40°...
Embodiment 2
[0055] Example 2: Synthesis of Fmoc-Cys(Mmt)-OH
[0056] 200g cysteine hydrochloride (Cys.HCl) add 1.5LN,N-dimethylformamide (DMF) to dissolve, slowly add 392g 4-methoxytriphenylchloromethane (Mmt-Cl) under stirring, After 4-methoxytriphenylchloromethane was added, the reaction was stirred at room temperature for 12 hours. Add 3L of water to the reaction solution under stirring, then add sodium carbonate (Na 2 CO 3 ) Adjust PH=6~7, precipitate solid, wash the solid with water 3 times (1.5L each time), filter, and then wash 3 times with ethyl acetate (1.5L each time), filter, and dry at 45°C to obtain 412g product Cys(Mmt ) (Yield 82.5%).
[0057] 400gCys(Mmt) plus 1L water (H 2 O), 0.5L tetrahydrofuran (THF) dissolved, add sodium carbonate (Na 2 CO 3 ) Adjust PH=7~9, add 342g Fmoc-OSu, stir and react for 12 hours, add sodium carbonate (Na 2 CO 3 ) Maintain the pH of the reaction solution at 7-9. After the reaction is complete, add 6N aqueous hydrochloric acid to adjust the pH to...
Embodiment 3
[0058] Example 3: Peptide resin 1: Synthesis of Cys(Mmt)-Resin
[0059] Weigh 184.7g of Fmoc-Cys(Mmt)-OH and 48.6g of HOBt, add 1600mL of DMF and stir to dissolve, add 48.8ml of DIC under stirring with ice-water cooling, and react for 0.5 hours to obtain an activated ester solution of protected amino acids for use. Weigh 158.7g (100mmol) of RinkAMResin with a substitution value of 0.63mmol / g, add it to the solid phase reactor, add 1600ml DMF and stir the swelling resin for 30 minutes, drain it, add 20% PIP / DMF solution (volume ratio) 1600mL, stir to react 0.5 After hours, drain it, wash it with DMF 6 times, and drain it to get RinkAMResin with Fmoc group removed. Add the activated ester solution of the protected amino acid to the Fmoc group-removed RinkAMResin, stir the reaction at room temperature, check the reaction with ninhydrin, and monitor every 1 hour until it is complete (the ninhydrin detection resin is colorless), and the reaction After completion, it was washed 6 time...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com