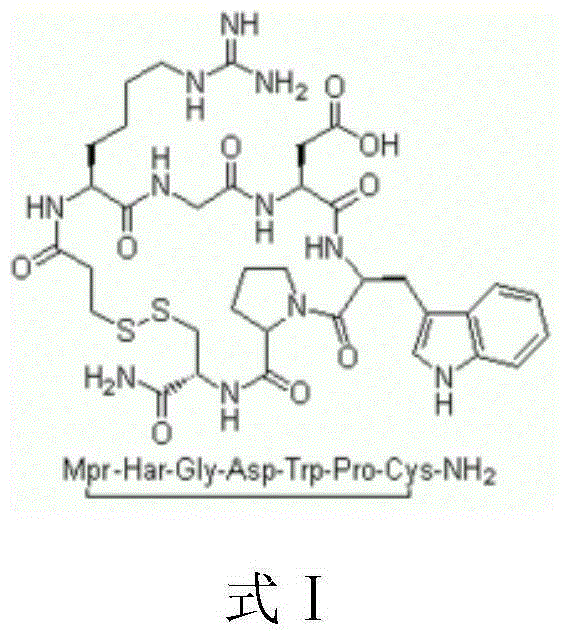
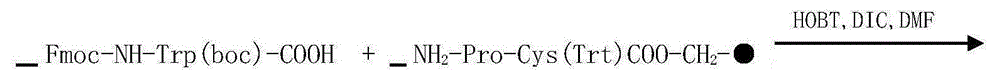Preparation method for eptifibatide
A technology for eptifibatide and peptide fragments, which is applied in the field of polypeptide drug preparation, can solve the problems of reducing the purification time and generating cost, prolonging the generating time, and low water-phase concentration efficiency, etc., so as to shorten the production process steps, reduce the purification difficulty, and achieve a wide range of The effect of practical value and application prospect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] The preparation of step 1Fmoc-Pro-Cys (Trt) resin
[0054] Add Fmoc-Cys(Trt) carrier resin (0.45mmol / g, 50mmol) into the reactor, wash once with DMF, and swell with DCM for 30 minutes. After swelling, 20% PIP / DMF solution was used to deprotect Fmoc for 2 times. After deprotection was detected, DMF was washed 3 times, and DCM was washed 3 times. 50.6g Fmoc-Pro-OH, (150mmol), 20.3gHOBt (150mmol), 19gDIC were dissolved in DMF, added to a solid-phase reactor, and reacted at room temperature for 3h (the end point of the reaction was determined by the ninhydrin method), and the reaction was completed. Wash 3 times with DMF and 3 times with DCM.
[0055] Preparation of step 2Fomc-Trp(boc)-Pro-Cys(Trt) resin
[0056] In the Fmoc-Pro-Cys (Trt) resin in step 1, 20% PIP / DMF solution was used to deprotect Fmoc twice, and after the deprotection was detected, wash three times with DMF and three times with DCM. Dissolve 78.9gFmoc-Trp(boc)-OH(150mmol), 20.3gHOBt(150mmol), and 19gDIC...
Embodiment 2
[0071] The preparation of step 1Fmoc-Pro-Cys (Trt) resin
[0072] Add Fmoc-Cys(Trt) carrier resin (0.45mmol / g, 50mmol) into the reactor, wash once with DMF, and swell with DCM for 30 minutes. After swelling, 20% PIP / DMF solution was used to deprotect Fmoc for 2 times. After deprotection was detected, DMF was washed 3 times, and DCM was washed 3 times. 50.6g Fmoc-Pro-OH, (150mmol), 20.3gHOBt (150mmol), 19gDIC were dissolved in DMF, added to a solid-phase reactor, and reacted at room temperature for 3h (the end point of the reaction was determined by the ninhydrin method), and the reaction was completed. Wash 3 times with DMF and 3 times with DCM.
[0073] Preparation of step 2Fomc-Trp(boc)-Pro-Cys(Trt) resin
[0074] In the Fmoc-Pro-Cys (Trt) resin in step 1, 20% PIP / DMF solution was used to deprotect Fmoc twice, and after the deprotection was detected, wash three times with DMF and three times with DCM. Dissolve 78.9gFmoc-Trp(boc)-OH(150mmol), 20.3gHOBt(150mmol), and 19gDIC...
Embodiment 3
[0089] The preparation of step 1Fmoc-Pro-Cys (Trt) resin
[0090] Add Fmoc-Cys(Trt) carrier resin (0.45mmol / g, 50mmol) into the reactor, wash once with DMF, and swell with DCM for 30 minutes. After swelling, 20% PIP / DMF solution was used to deprotect Fmoc for 2 times. After deprotection was detected, DMF was washed 3 times, and DCM was washed 3 times. 50.6g Fmoc-Pro-OH, (150mmol), 20.3gHOBt (150mmol), 19gDIC, 0.5g triethylamine and 0.45g N-ethyl p-toluidine were dissolved in DMF, added to a solid-phase reactor, and reacted at room temperature for 3h ( The end point of the reaction is determined by the ninhydrin method), and the reaction is completed, washed 3 times with DMF and 3 times with DCM.
[0091] Preparation of step 2Fomc-Trp(boc)-Pro-Cys(Trt) resin
[0092] In the Fmoc-Pro-Cys (Trt) resin in step 1, 20% PIP / DMF solution was used to deprotect Fmoc twice, and after the deprotection was detected, wash three times with DMF and three times with DCM. Dissolve 78.9gFmoc-T...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com